Preparation method of efficient water-resistant copper-doped manganese-based catalyst and application thereof in low-concentration ozone decomposition
A technology of ozone decomposition and catalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., which can solve the limitation of wide application of ozone decomposition catalyst and low efficiency of ozone decomposition , complex preparation process and other issues, to achieve good catalytic ozone degradation effect, wide application range, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
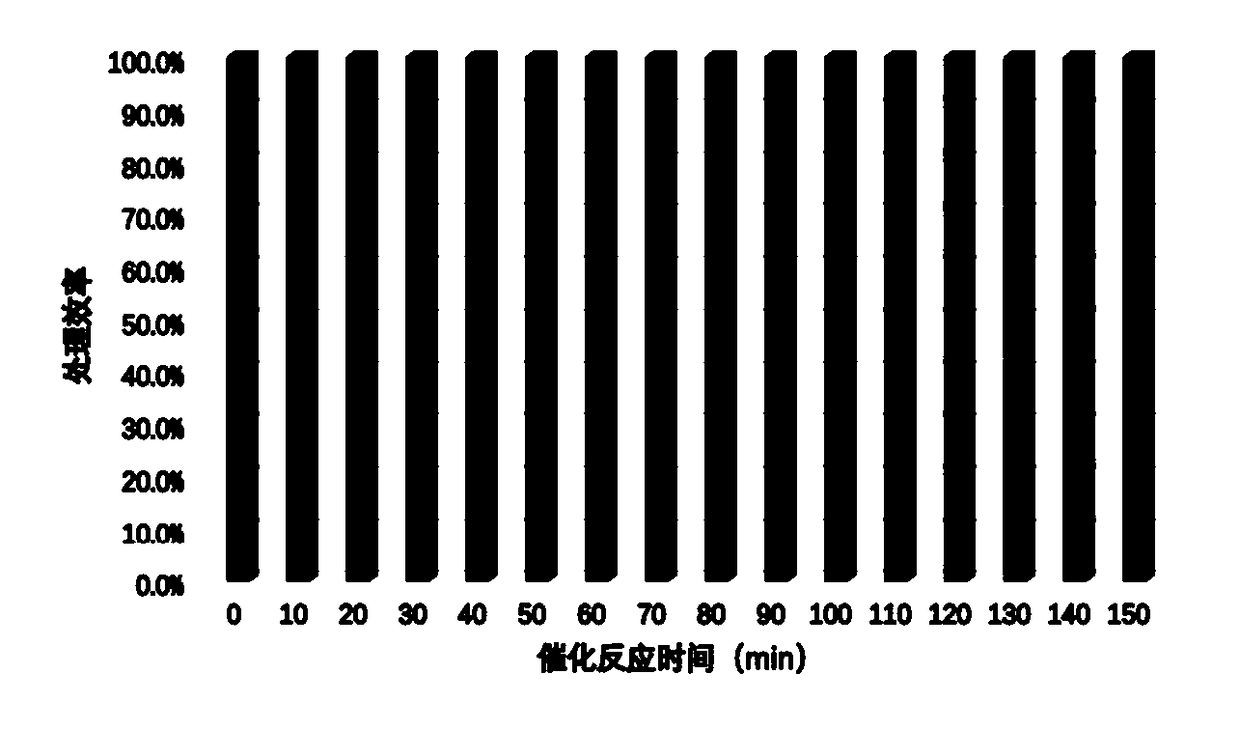
[0020] Dissolve 0.474g of potassium permanganate in 15mL of deionized water and stir for 30min to form homogeneous phase A. Dissolve 1.104 g of manganese acetate in 15 mL of deionized water and stir for 30 min to form a homogeneous phase B. Then mix liquid A and liquid B evenly under continuous stirring, then add 0.0096g of copper nitrate solid to the uniformly mixed solution, transfer it to a 150mL hydrothermal reaction kettle after ultrasonic dissolution, and adjust the temperature to 140°C for 2 hours , naturally cooled after the reaction. The precipitate was obtained by centrifugation, washed several times with deionized water and dried overnight. Then put the catalyst material in 2mol / L hydrochloric acid for acid treatment for 15min, and dry it for later use. Add 1ml of PTFE emulsion (60%) to deionized water at a ratio of 1:30, then add 200mg of the above-mentioned catalyst powder to the diluted emulsion, and stir ultrasonically for 30min to obtain an impregnation solut...
Embodiment 2
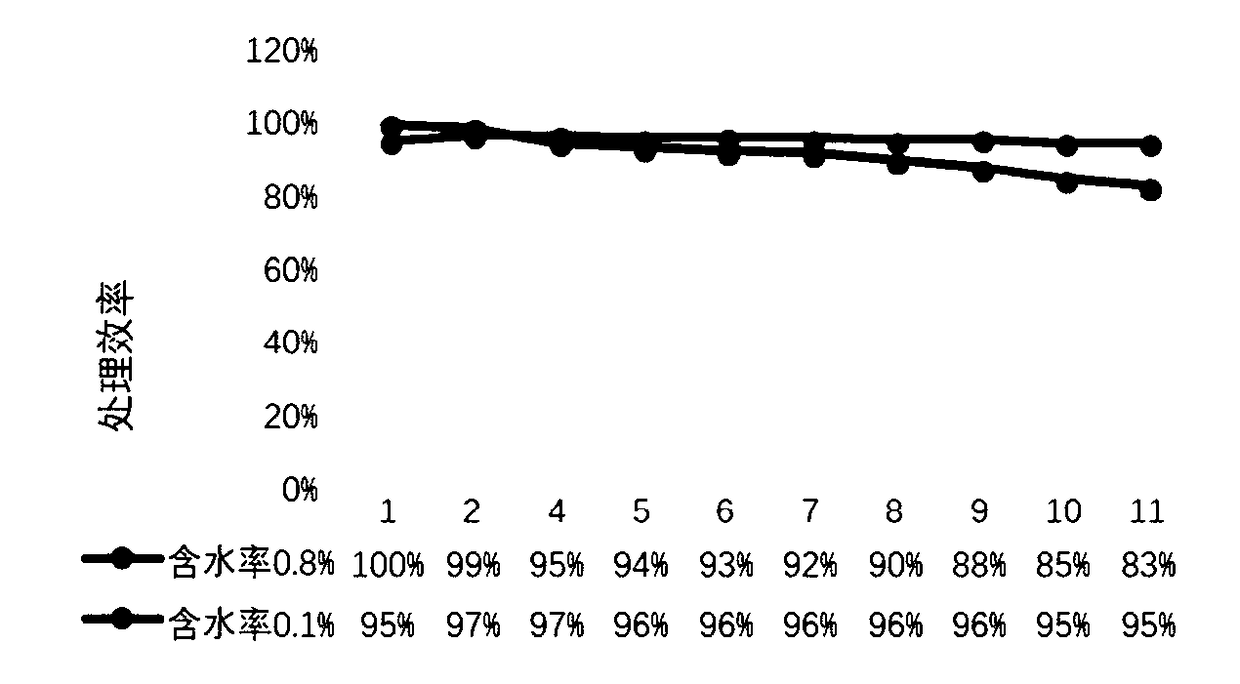
[0022] Dissolve 1.58g of potassium permanganate in 50mL of deionized water and stir for 30min to form homogeneous phase A. Dissolve 3.68g of manganese acetate in 50mL of deionized water and stir for 30min to form a homogeneous phase B. Then mix liquid A and liquid B evenly under continuous stirring, then add 0.032g of copper nitrate solid to the uniformly mixed solution, transfer it to a 150mL hydrothermal reaction kettle after ultrasonic dissolution, and adjust the temperature to 140°C for 2 hours , naturally cooled after the reaction. The precipitate was obtained by centrifugation, washed several times with deionized water and dried overnight. Then the catalyst material was placed in 5mol / L hydrochloric acid for acid treatment for 15min, and dried for later use. Add 1ml of PTFE emulsion (60%) to deionized water at a ratio of 1:30, then add 200mg of the above-mentioned catalyst powder to the diluted emulsion, and stir ultrasonically for 30min to obtain an impregnation solut...
Embodiment 3
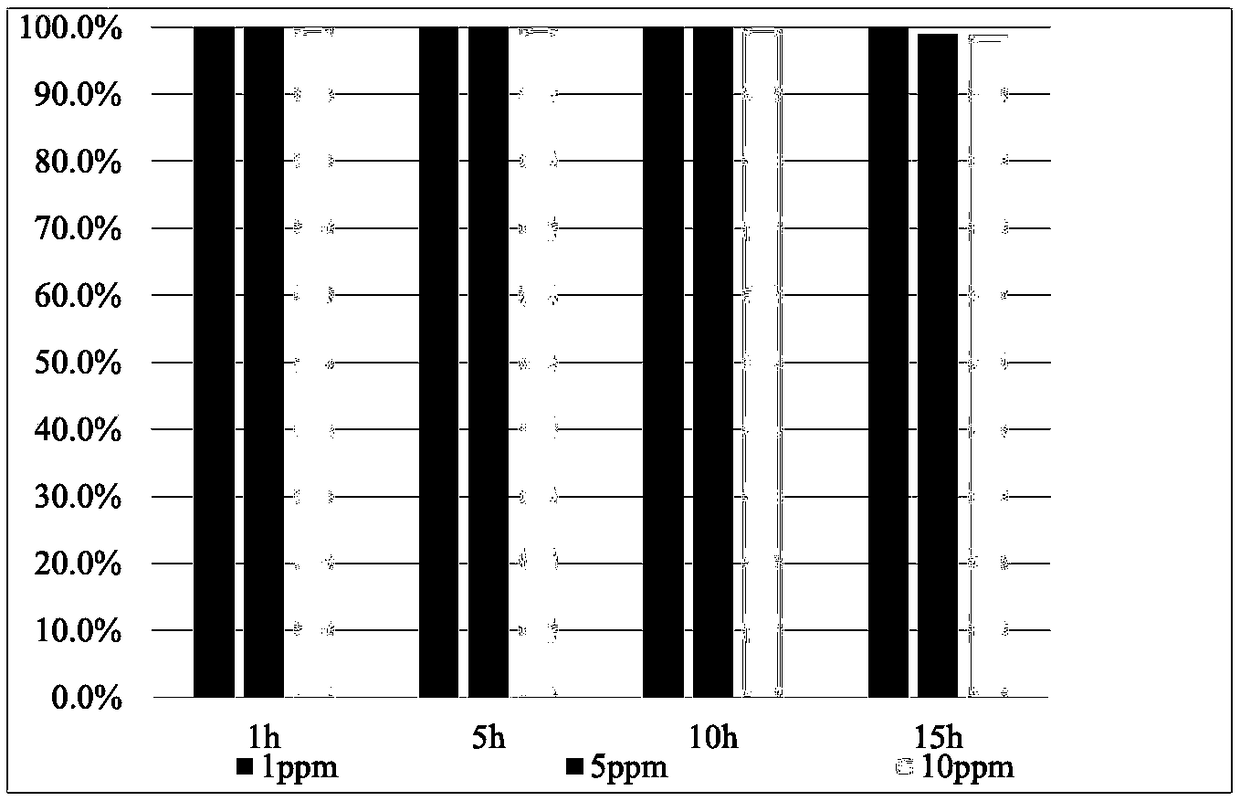
[0024] Dissolve 1.58g of potassium permanganate in 50mL of deionized water and stir for 30min to form homogeneous phase A. Dissolve 3.68g of manganese acetate in 50mL of deionized water and stir for 30min to form a homogeneous phase B. Then mix liquid A and liquid B evenly under continuous stirring, then add 0.032g of copper nitrate solid to the uniformly mixed solution, transfer it to a 150mL hydrothermal reaction kettle after ultrasonic dissolution, and adjust the temperature to 160°C for 4 hours , naturally cooled after the reaction. The precipitate was obtained by centrifugation, washed several times with deionized water and dried overnight. Then put the catalyst material in 2mol / L hydrochloric acid for acid treatment for 30min, and dry it for later use. Add 1ml of PTFE emulsion (60%) to deionized water at a ratio of 1:30, then add 200mg of the above-mentioned catalyst powder to the diluted emulsion, and stir ultrasonically for 30min to obtain an impregnation solution. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com