Detection and analysis method for quality control of raltitrexed synthesis
A technology of raltitrexed and analysis method, which is applied in the detection and analysis field of raltitrexed synthetic quality control, to achieve the effects of comprehensive quality control, convenient operation and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 High performance liquid chromatography determines the purity of starting material 6-bromomethyl-3,4-dihydro-2-methyl-quinazolin-4-one
[0044] 1.1 Chromatographic conditions
[0045] Instrument: Agilent 1260 high performance liquid chromatography;
[0046] Detector: VWD;
[0047] Chromatographic column: Agilent Extend-C18 chromatographic column (4.6mm×250mm, 5μm);
[0048] Mobile phase: water: acetonitrile = 80:20;
[0049] Column temperature: 35°C;
[0050] Flow rate: 1.0mL / min;
[0051] Detection wavelength: 230nm;
[0052] Injection volume: 10μL
[0053] 1.2 Sample preparation
[0054] Blank solution: 50% acetonitrile in water
[0055] Preparation of positioning solution: Accurately weigh about 10 mg of impurity E and impurity X, add appropriate amount of 50% acetonitrile aqueous solution to dissolve, and dilute to make a solution containing 0.1 mg per 1 mL, respectively as impurity positioning solution.
[0056] Preparation of system suitabilit...
Embodiment 2
[0074] Embodiment 2 HPLC measures the purity of starting material 5-nitro-2-thiophenecarboxylic acid
[0075] 2.1 Chromatographic conditions
[0076] Instrument: Agilent 1260 high performance liquid chromatograph
[0077] Detector: VWD
[0078] Workstation: Agilent OpenLAB CDS (EZChrom Edition)
[0079] Chromatographic column: Agilent Extend-C18 (4.6mm×250mm, 5μm)
[0080] Detection wavelength: 226nm
[0081] Flow rate: 1.0mL / min
[0082] Column temperature: 30°C
[0083] Injection volume: 10μL
[0084] Mobile phase: 0.1% trifluoroacetic acid in water: methanol (55:45)
[0085] 2.2 Sample preparation
[0086] Blank: 45% methanol in water
[0087] 5-Nitro-2-thiophene formaldehyde stock solution: Accurately weigh about 10.0mg of 5-nitro-2-thiophene formaldehyde, put it in a 20mL measuring bottle, add a blank solvent to dissolve and dilute to the mark, shake well to get ready. (about 0.5mg / mL)
[0088] 4-Nitro-2-thiophenecarboxylic acid stock solution: Accurately weigh...
Embodiment 3
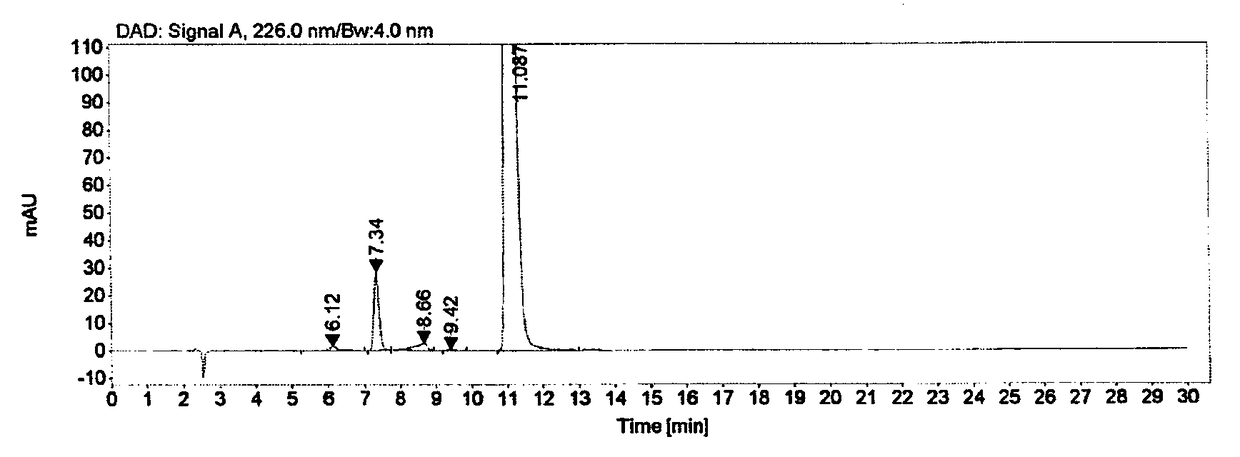
[0107] Embodiment 3 high performance liquid chromatography is determined the content of raltitrexed
[0108] 3.1 Chromatographic conditions
[0109] Instrument: Agilent 1260 high performance liquid chromatograph
[0110] Detector: DAD
[0111] Workstation: Agilent OpenLAB CDS (EZChrom Edition)
[0112] Chromatographic column: Agilent Extend-C18 (4.6mm×250mm, 5μm)
[0113] Detection wavelength: 226nm
[0114] Flow rate: 1.0mL / min
[0115] Column temperature: 30°C
[0116]Injection volume: 10μL
[0117] Mobile phase: buffer-methanol (70:30), where the buffer is 0.005mol / L tetrabutylammonium bromide aqueous solution (adjust the pH to 8.5 with phosphoric acid)
[0118] 3.2 Solution preparation
[0119] Reference substance solution: take a certain amount of raltitrexed reference substance, and prepare a solution with a concentration of about 1.0 mg / mL with 0.1 mol / L sodium hydroxide aqueous solution.
[0120] Test product solution: take a certain amount of raltitrexed test...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com