Escherichia coli recombinant strain for heterologous expression of nitrile hydratase and application thereof
A technology of Escherichia coli and nitrile hydratase, applied in the field of bioengineering, can solve the problems of low production efficiency, long growth cycle, difficult product purification, etc., and achieve the effects of high production efficiency, short fermentation cycle, and simplified separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Construction of recombinant Escherichia coli BL21 / pET24a-PtNHase
[0045] The NHase gene sequence derived from Pseudonocardia thermophila was retrieved through NCBI, optimized through the codon online optimization website Graphical Codon Usage Analyzer, and a spacer sequence was added between the nhhB and nhhA genes, nhhA and nhhP genes, specifically: the encoded amino acid sequence The nhhB gene of SEQ ID NO.1, the spacer sequence a shown in SEQ ID NO.4, the nhhA gene of the encoded amino acid sequence SEQ ID NO.2, the spacer sequence b shown in SEQ ID NO.5, the encoded amino acid sequence SEQ The nhhP gene of ID NO.3 is sequentially connected to obtain the gene NHase of nitrile hydratase, the nucleotide sequence is shown in SEQ ID NO.6, and the electrophoresis result is shown in figure 1 Shown, synthesized by General Biosystems (Anhui) Co., Ltd.
[0046] The optimized NHase sequence did not contain any restriction endonuclease sites. Specific primers P1 ...
Embodiment 2
[0050] The expression of embodiment 2 nitrile hydratase
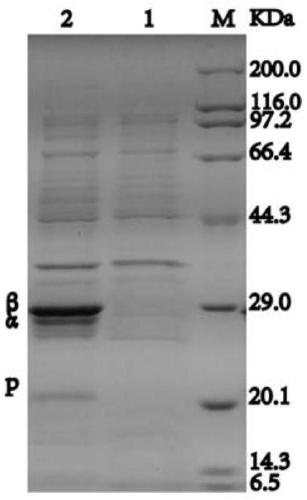
[0051] Recombinant Escherichia coli BL21 / pET24-PtNHase was inoculated in 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, and cultured overnight at 37°C with shaking at 200 r / min. Inoculate the above-mentioned overnight culture into LB medium containing 100 μg / mL of kanamycin at an inoculum size of 1%, and culture it with shaking at 37°C and 200 r / min until the bacterial liquid OD 600 To 0.6-0.8, add IPTG to a final concentration of 0.4mmol / L, induce culture at 20°C for 16-20h, collect the bacteria and ultrasonically disrupt them, analyze and identify the expression level of nitrile hydratase recombinant protein by Tris-tricine SDS-PAGE method, the results are as follows image 3 shown. After sonication and centrifugation at 12000rpm for 60min, the protein was purified with affinity chromatography column Strep Trap FF, and the specific enzyme activity of the pure recombinant nitrile hydratase was 907.69U / ...
Embodiment 3
[0052] Embodiment 3 Recombinant escherichia coli high-density fermentation
[0053] Recombinant Escherichia coli BL21 / pET24-PtNHase was inoculated in 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, and cultured overnight at 37°C with shaking at 200 r / min. The above-mentioned overnight culture was inoculated into LB medium containing 100 μg / mL of kanamycin at an inoculum amount of 1%, and cultured with shaking at 37° C. and 200 r / min for 6-8 hours. The above-mentioned culture was inoculated into 2L fermenter fermentation medium containing kanamycin concentration of 100 μg / mL according to the inoculum size of 6%, fed culture at 37 ° C, when OD 600 When it reaches 60°C, the temperature is lowered to 30°C, and 140-150mL of inducer is added at a constant rate of 0.20-0.22g / (L·h), and the induction culture is 36h to end the fermentation. After the fermentation, the enzyme activity reached 24763.48U / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com