Synthesis method of commercial BODIPY
A synthesis method and compound technology, applied in the field of fluorescent dye synthesis, can solve the problems of long synthesis steps, low synthesis yield, serious environmental pollution, etc., and achieve the effect of reducing synthesis cost and high-efficiency synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
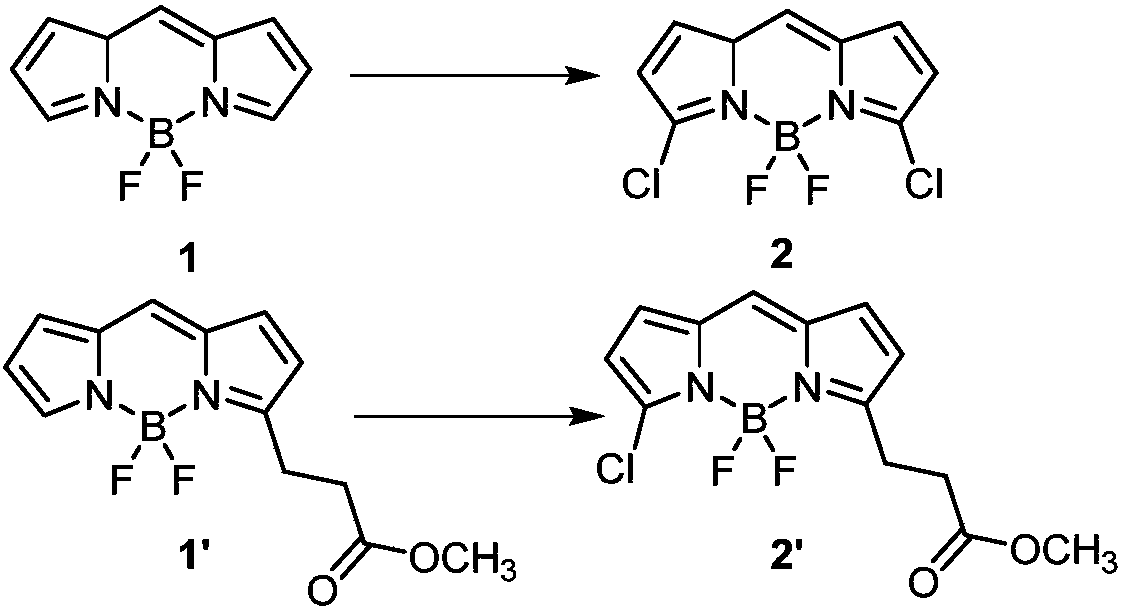
[0030] 1. Dissolve 1.03g (5.3mmol) of compound 1 in 60mL of acetonitrile, add 0.91g (21.2mmol) of lithium chloride, use an electrosynthesizer, constant current mode, 3mA current and stir at room temperature for 8h. After the reaction was finished, the reaction solution was transferred to a round bottom flask and concentrated under reduced pressure, and then purified by column chromatography to obtain 0.92g of yellow solid compound 2 with a yield of 66%. The structural characterization data were: 1 H NMR (600MHz, CHCl 3 -d) δ: 7.13(s, 1H), 7.07(d, J=4.2Hz, 2H), 6.42(d, J=4.4Hz, 2H); 13 C NMR (151MHz, CDCl 3 )δ: 145.7, 134.1, 131.4, 127.9, 119.1.
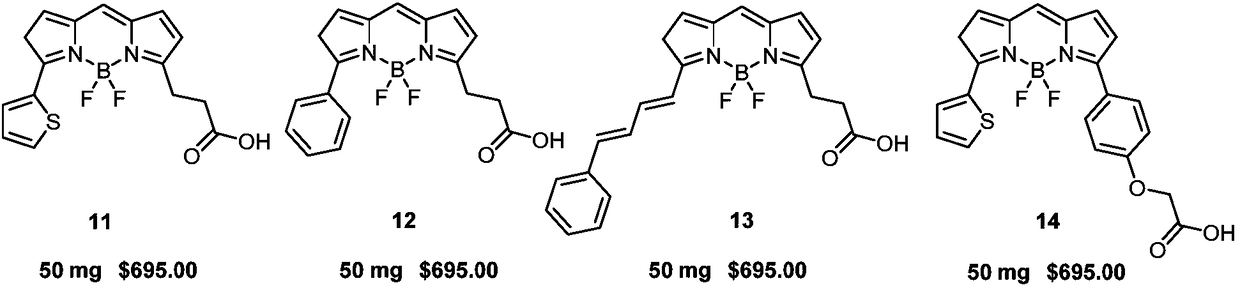
[0031] 2. Dissolve 200mg (0.76mmol) of compound 2 in 5mL of toluene, add 1mL of 2.5mol / L (2.5mmol) sodium carbonate aqueous solution, 43mg (0.04mmol) tetrakistriphenylphosphopalladium, 145mg (1.14mmol) thiophene-2 - Boric acid 3-1, heat the reaction solution to reflux for 5 hours under the protection of nitrogen, after ...
Embodiment 2
[0036]
[0037]1. Compound 2 was synthesized according to the method in Step 1 of Example 1.
[0038] 2. Compound 5-1 was synthesized according to the method in Step 2 of Example 1.
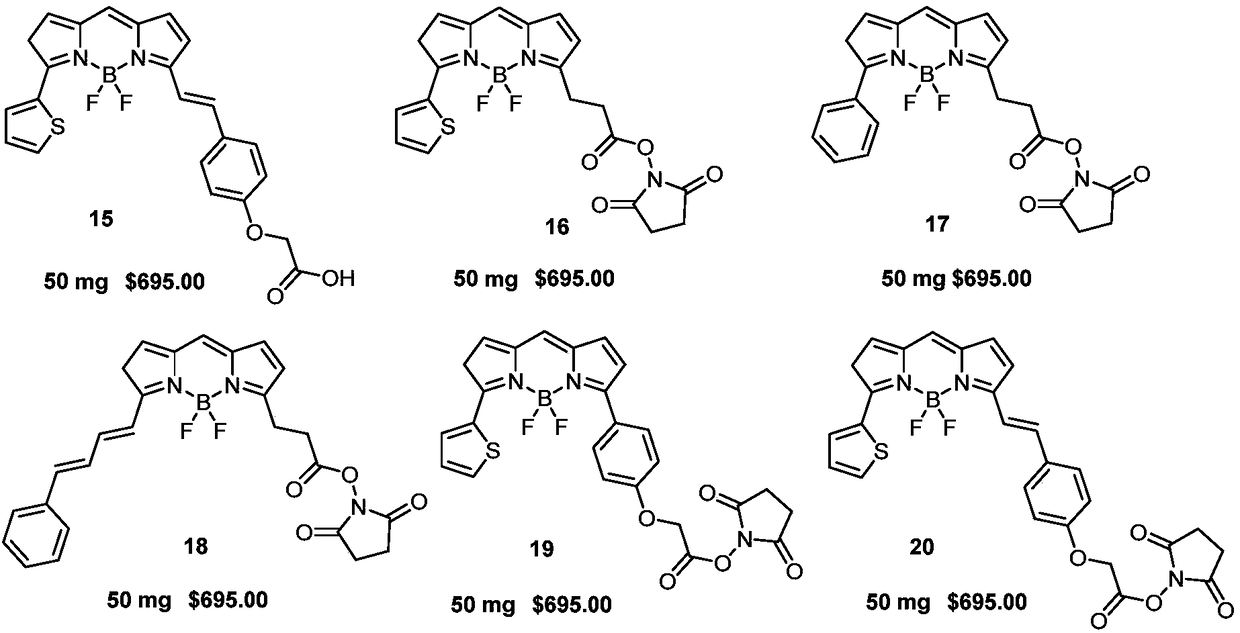
[0039] Dissolve 130mg (0.42mmol) of compound 5-1 in 5mL of toluene, add 0.3mL 2.5mol / L (0.75mmol) sodium carbonate aqueous solution, 24.3mg (0.03mmol) tetrakistriphenylphosphopalladium, 148.7mg (0.63mmol) (E)-(4-(2-methoxy-2-oxyethoxy)styryl)boronic acid 4-2, under the protection of nitrogen, the reaction solution was heated to reflux for 5h, after the reaction was completed, use a separatory funnel After extraction, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by column chromatography to obtain 143 mg of reddish-brown solid compound 5-3 with a yield of 73%. The structural characterization data are: 1 H NMR (600MHz, CHCl 3 -d) δ: 8.20(dd, J=3.8, 1.1Hz, 1H), 7.64-7.56(m, 3H), 7.47(dd, J=5.1, 1.1Hz, 1H), 7.32(d, J=16.3Hz ,1H),7.2...
Embodiment 3
[0043]
[0044] 1. Dissolve 1.2g (4.3mmol) of compound 1' in 60mL of acetonitrile, add 273mg (6.4mmol) of lithium chloride, use an electrosynthesizer, constant current mode, 2mA current and stir at room temperature for 10h. After the reaction was finished, the reaction solution was transferred to a round bottom flask and concentrated under reduced pressure, then purified by column chromatography to obtain 1.17g of tan solid compound 2' with a yield of 86%, and the structural characterization data were: 1 H NMR (600MHz, CHCl 3 -d) δ: 7.11(s, 1H), 7.06(d, J=4.3Hz, 1H), 6.97(d, J=4.2Hz, 1H), 6.43(d, J=4.3Hz, 1H), 6.35( d, J=4.2Hz, 1H), 3.69(s, 3H), 3.34(t, J=7.5Hz, 2H), 2.79(t, J=7.5Hz, 2H); 13 C NMR (151MHz, CDCl 3 ): δ172.5, 164.0, 142.1, 135.7, 133.2, 132.4, 129.3, 127.8, 120.3, 117.5, 51.8, 32.8, 24.3.
[0045] 2. Dissolve 300mg (0.96mmol) of compound 2' in 5mL of toluene, add 2mL of 2.5mol / L (5mmol) sodium carbonate aqueous solution, 55.5mg (0.05mmol) tetrakistriphenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com