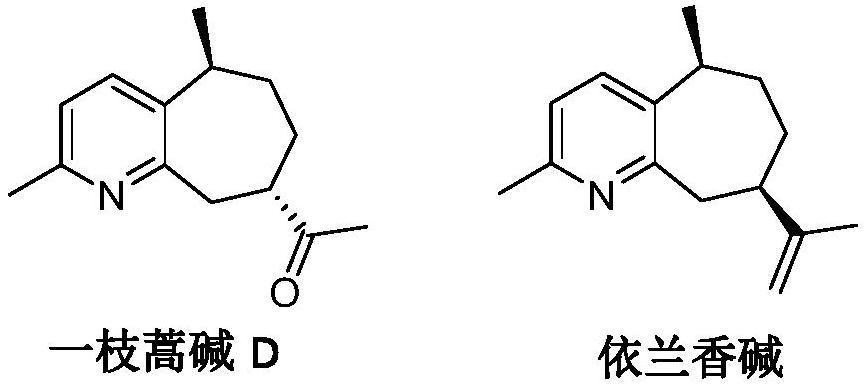
Method for preparing guaipyridine type sesquiterpenoid alkaloid
A technology for sesquiterpenoids and alkaloids, which is applied in the field of preparing guaiacol-type sesquiterpene alkaloids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
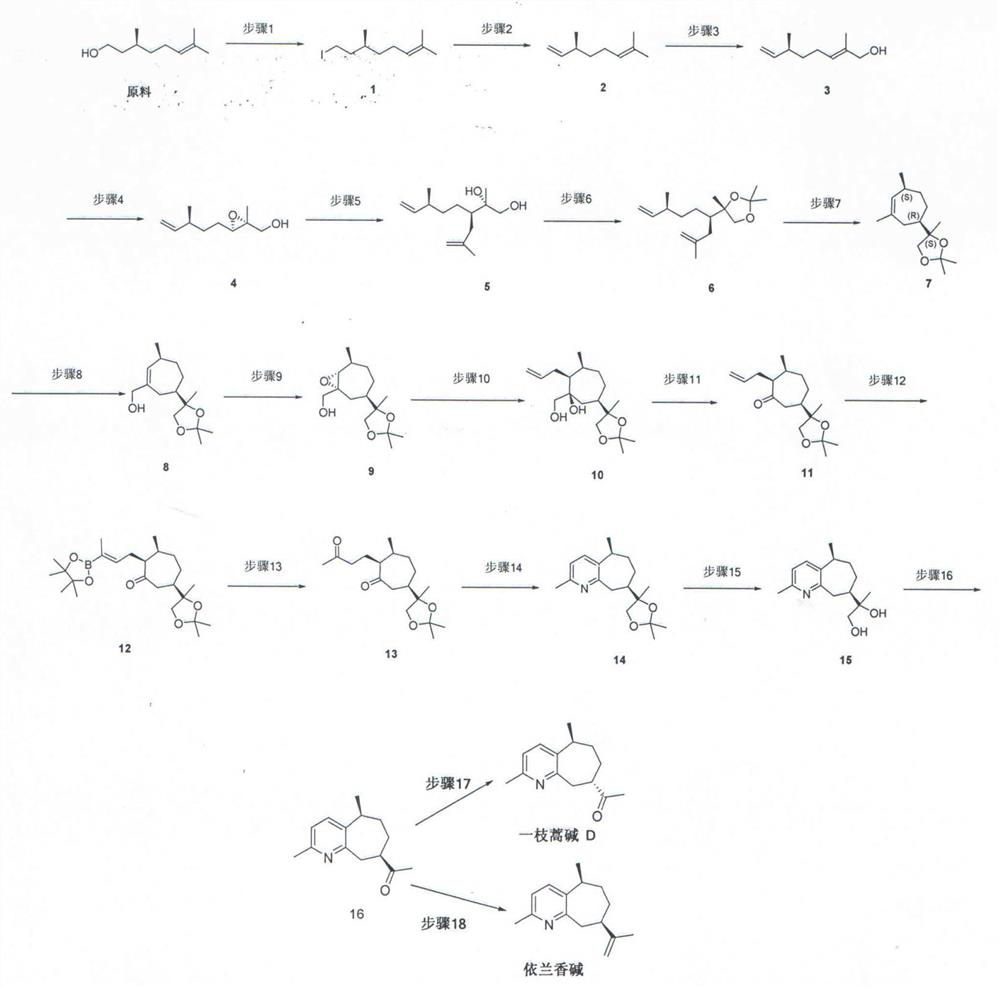
[0033] Preparation of compound 1
[0034] Dissolve the raw material citronellol 100g, 640mmol in 1.40L of dichloromethane, add 185g, 704mmol of triphenylphosphine at 0°C, add 95.9g, 1410mmol of imidazole after 5min, slowly add 179g, 704mmol of iodine in batches after 30min, React at room temperature for 5 hours, add saturated sodium thiosulfate solution to quench, dichloromethane was evaporated to dryness, petroleum ether was added, pulped, filtered with suction, the filtrate was evaporated to dryness, and purified to obtain compound 1 named (S)-8-iodo-2, 6-Dimethyloct-2-ene 162g, yield 95%;
[0035] 1 H NMR (400MHz, CDCl 3 )δ5.11–5.07(m,1H),3.29–3.20(m,1H),3.20–3.08(m,1H),2.09–1.80(m,3H),1.68(s,3H),1.67–1.62( m,1H),1.60(s,3H),1.59–1.48(m,1H),1.40–1.26(m,1H),1.22–1.12(m,1H),0.89(d,J=6.6Hz,3H) .
Embodiment 2
[0037] Preparation of compound 2
[0038] Compound 1 obtained in Example 1 was (S)-8-iodo-2,6-dimethyloct-2-ene 162g, 610mmol was dissolved in 1.50L tetrahydrofuran, and potassium tert-butoxide 75.3 g, 671mmol, reacted overnight, quenched by adding saturated ammonium chloride, extracted, concentrated, and purified to obtain compound 2 named (S)-3,7-dimethyloctadiene-1,6-diene, also known as Citronellene 80.9g, yield 96%;
[0039] 1 H NMR (400MHz, CDCl 3 )δ5.75–5.66(ddd,1H),5.13–5.08(m,1H),4.98–4.90(m,2H),2.16–2.09(m,1H),2.00–1.93(m,2H),1.69( s, 3H), 1.60 (s, 3H), 1.35–1.29 (m, 2H), 0.99 (d, J=6.8Hz, 3H).
Embodiment 3
[0041] Preparation of compound 3
[0042] 20.4g of selenium dioxide, 148mmol, 42g of tert-butanol peroxide, 326mmol, and 70% aqueous solution were dissolved in 600mL of dichloromethane, reacted at room temperature for 30 minutes, and then added the compound 2 obtained in Example 2 to be (S)-3, 7-Dimethyloctadiene-1,6-diene, continued to react for 2 hours, quenched by adding saturated sodium thiosulfate, extracted, concentrated, and purified to obtain compound 3 named (S, E)-2,6 -Dimethyloctyl-2,7-dien-1-ol 15.9g, yield 70%;
[0043] 1 H NMR (400MHz, CDCl 3 )δ5.67(ddd,J=17.5,10.3,7.5Hz,1H),5.38–5.34(m,1H),4.96–4.88(m,2H),3.95(d,J=6.0Hz,2H),2.14 -2.07 (m, 1H), 2.05 - 1.92 (m, 3H), 1.63 (s, 2H), 1.32 (qd, J=7.2, 1.5Hz, 2H), 0.97 (d, J=6.8Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com