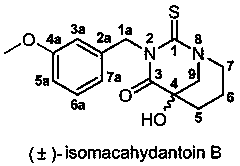
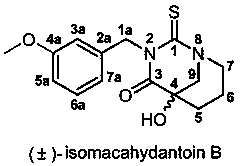
Bionic synthesis and preparation method of (+/-)-isomacahydantoin B
A biomimetic synthesis, thiourea technology, applied in the direction of organic chemistry, can solve the problems of limited biological activity research, structural identification errors, lack and other problems, and achieve the effect of simple and efficient method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
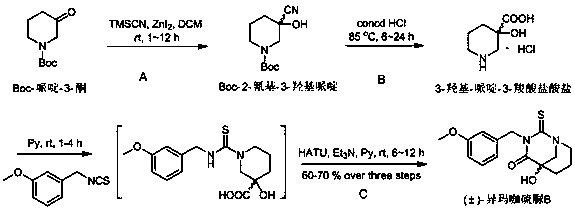
[0032] The preparation method of (±)-isomacathiourea B uses Boc-piperidin-3-one as the starting material for the reaction, and then performs nucleophilic reaction with trimethylsilylcyanide (TMSCN) catalyzed by zinc iodide Substitution reaction to obtain Boc-2-cyano-3-hydroxypiperidine (first step reaction A), and then hydrolysis by concentrated hydrochloric acid to obtain 3-hydroxy-piperidine-3-carboxylate hydrochloride (second step reaction B ), and finally (±)-isomacathiourea B was obtained by Edman reaction with 3-methoxybenzyl isothiocyanate (third step reaction C). The yield of this three-step reaction route is as high as 60~70%, and it can achieve gram-level preparation. The specific route is:
[0033]
[0034] (A) Preparation of Boc-2-cyano-3-hydroxypiperidine: Boc-piperidin-3-one (0.199~19.9 g, 1.0~100.0mmol) was dissolved in 5~500 mL of anhydrous dichloromethane solution , under nitrogen protection, fully stirred and dissolved, added zinc iodide (0.318~31.8 g, 1.0~...
Embodiment 1
[0039]
[0040] Boc-piperidin-3-one (0.398 g, 2.0 mmol) was dissolved in 10 mL of anhydrous dichloromethane solution, under nitrogen protection, after fully stirring and dissolving, zinc iodide (0.636 g, 2.0 mmol ) and trimethylsilylcyanide (0.396g, 3.0 mmol), stirred at room temperature for 6 hours. The reaction solution was filtered with a suction filter bottle, and after zinc iodide was filtered out, it was evaporated to dryness with a rotary evaporator under reduced pressure. After vacuum filtration, it was dried in a vacuum oven for 12 hours to obtain Boc-2-cyano-3-hydroxypiperone A sample of pyridine (0.566 g, about 80% pure) yielded about 90%. The obtained Boc-2-cyano-3-hydroxypiperidine (0.258 g, 2.0 mmol) was dissolved in 15 mL of concentrated hydrochloric acid solution, and refluxed at 85°C for 12 hours. The reaction solution was evaporated to dryness under reduced pressure with a rotary evaporator, filtered under reduced pressure and dried in a vacuum oven for 1...
Embodiment 2
[0042]
[0043] Boc-piperidin-3-one (1.990 g, 10.0 mmol) was dissolved in 50 mL of anhydrous dichloromethane solution, under nitrogen protection, after fully stirring and dissolving, zinc iodide (3.180 g, 10.0 mmol ) and trimethylsilylcyanide (1.980 g, 15.0 mmol), stirred at room temperature for 6 hours. The reaction solution was filtered with a suction filter bottle, and after zinc iodide was filtered out, it was evaporated to dryness with a rotary evaporator under reduced pressure, and after vacuum filtration, it was dried in a vacuum oven for 6 hours to obtain Boc-2-cyano-3-hydroxypiperol A sample of pyridine (2.830 g, about 80% pure) yielded about 90%. The obtained Boc-2-cyano-3-hydroxypiperidine (1.290 g, 10.0 mmol) was dissolved in 45 mL of concentrated hydrochloric acid solution, and refluxed at 85 degrees Celsius for 12 hours. The reaction solution was evaporated to dryness with a rotary evaporator, filtered under reduced pressure and dried in a vacuum oven for 12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com