Synthetic method of boroazaphenanthrene and derivatives thereof
A synthetic method and a phenanthrene technology, which is applied in the field of synthesis of borazaphenanthrene and its derivatives, can solve the problems that the potential application characteristics have not been widely developed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
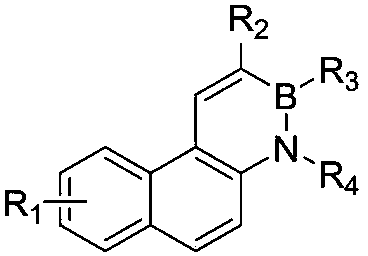
[0033] A kind of overall synthetic method of borazine phenanthrene and its derivatives of the present invention comprises the following synthetic routes and steps:
[0034]
[0035] Some of the above-mentioned compounds are given examples, and the details are as follows:
Embodiment 1
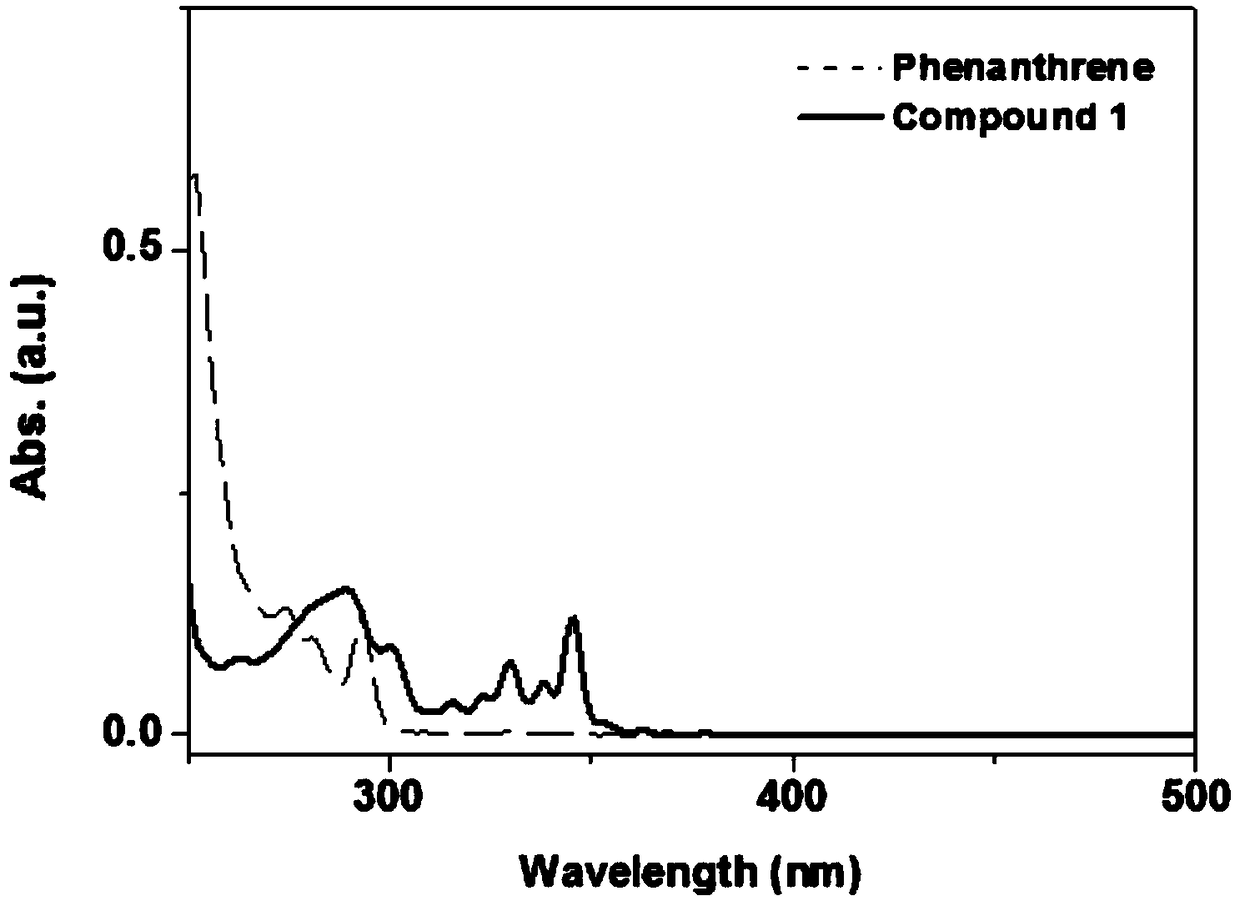
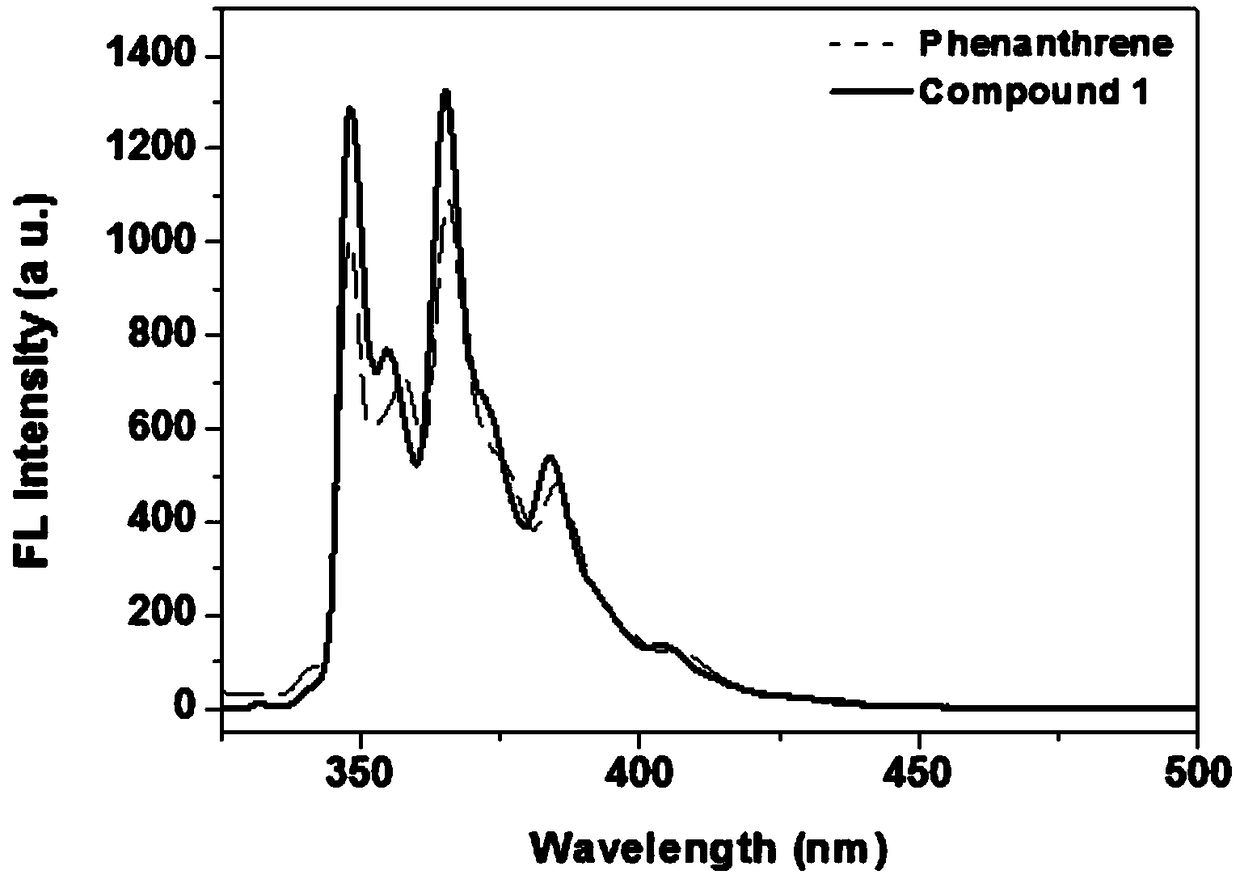
[0036] Embodiment 1: the synthesis of 1,2-azaboraphenanthrene compound 1
[0037] 1) Synthesis of compound 4: Weigh 2-nitro-1-naphthol (1.0equiv, 27mmol, 5.07g) and add trifluoromethanesulfonic anhydride (1.25equiv, 34mmol, 9.5g) at 0°C, triethylamine (2.4equiv, 65mmol, 6.5g) was stirred at room temperature for 12h. After the reaction was complete, water and dichloromethane were added for extraction, the organic layers were combined, and dried over anhydrous magnesium sulfate, filtered, spin-dried, and quickly separated by column chromatography to obtain the target compound as a yellow solid (7.4 g, yield 85%);
[0038] 1 HNMR (400MHz, CDCl 3 ):δ8.26-8.30(m,1H,Ar),8.08(d,J=9.2Hz,1H,Ar),7.98-8.02(m,2H,Ar),7.78-7.83(m,2H,Ar) .
[0039] 2) Synthesis of Compound 5: Weigh 2-nitro-1-trifluoromethanesulfonic naphthalene (1.0equiv, 18.4mmol, 5.93g), tetrakistriphenylphosphine palladium (0.1equiv, 1.8mmol, 2.08g) , sodium carbonate (5.0equiv, 91.8mmol, 9.7g) was pumped for three t...
Embodiment 2
[0045] Embodiment 2: Synthesis of 1,2-azaboraphenanthrene derivatives
[0046] 5) Synthesis of Compound 12: Weigh 2-amino-1-vinylnaphthalene (1.0equiv, 5.0mmol, 850mg), add chlorobenzene in the glove box and stir to dissolve, then add triethylamine (1.5equiv, 7.5mmol, 1.05mL) and dichlorophenylborane (1.25equiv, 6.28mmol, 0.68mL) were placed at 130°C and heated to reflux for 12 hours. After the reaction was completed, water and dichloromethane were added for extraction. The organic layers were combined and dried over anhydrous magnesium sulfate. Filtration, spin-drying, and separation by column chromatography gave the white target compound (1.27 g, yield 99%).
[0047] 1 HNMR (400MHz, CDCl 3 ):δ9.10(d,J=12.0Hz,1H,Ar),8.58(d,J=8.8Hz,1H,Ar),8.41(br,1H,NH),7.98(dd,J 1 =8.0Hz,J 2 =2.0Hz, 2H, Ar), 7.89(t, J=6.8Hz, 2H, Ar), 7.64-7.67(m, 1H, Ar), 7.45-7.52(m, 6H, Ar).
[0048] 6) Synthesis of compound 13: Weigh 2-phenyl-1,2-azaborine (1.0equiv, 1.37mmol, 350mg), add dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com