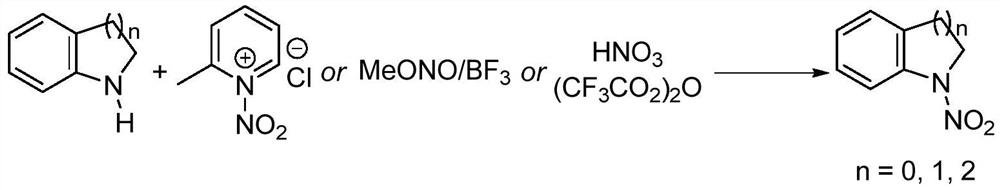A kind of synthetic method of nitramide
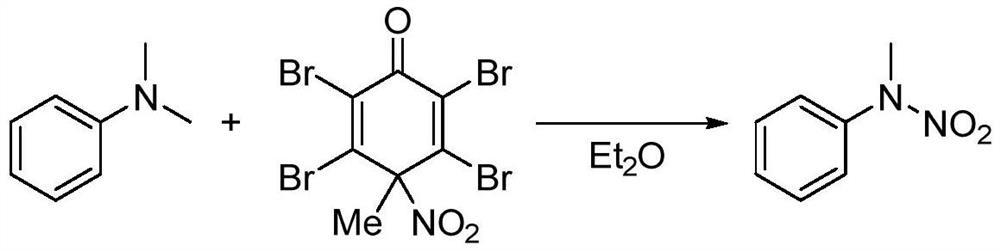
A synthesis method and technology of nitramide, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of poor stability, many reaction steps, high price, etc., and achieve high synthesis efficiency, high conversion rate, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
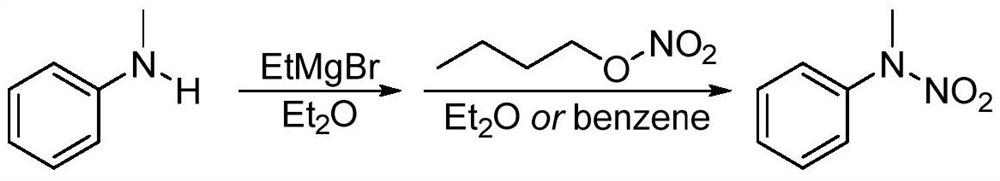
[0030] A kind of synthesis of N-methyl-N-phenyl nitramide:
[0031] ① Add 2.14g of N-methylaniline to a 50mL round bottom flask, add 25mL of acetonitrile, stir at room temperature to dissolve;
[0032] ② Slowly add 5.22 g of nitropyridinium salt to the reaction system in batches, and the ratio of the amount of substrate N-methylaniline to the nitropyridinium salt of the nitrating agent is 1:1.5;
[0033] ③React directly in an air atmosphere, mix well at room temperature, slowly heat to 50°C, monitor the progress of the reaction with thin-layer chromatography, react at 0.1MPa for 6 hours, the raw materials are completely converted, stop the reaction, and cool to room temperature;
[0034] ④Add 50mL saturated NaHCO to the system 3 solution, then add 50mL dichloromethane solution, shake, separate liquid, extract the aqueous phase with dichloromethane three times, 30mL each time, combine the organic phase, wash twice with saturated brine, 10mL each time, and concentra...
Embodiment 2
[0038]
[0039] A kind of synthesis of N-nitroindoline:
[0040] ① Add 2.38g of indoline to a 50mL round bottom flask, add 25mL of acetonitrile, stir at room temperature to dissolve;
[0041] ② Slowly add 4.18g of nitropyridinium salt to the reaction system in batches, and the ratio of the amount of substrate indoline to the nitropyridinium salt of the nitrating agent is 1:1.2;
[0042] ③React directly in the air atmosphere, mix well at room temperature, slowly heat to 40°C, monitor the progress of the reaction with thin-layer chromatography, react at 0.1MPa for 4.5h, the raw materials are completely converted, stop the reaction, and cool to room temperature;
[0043] ④Add 50mL saturated NaHCO to the system 3 solution, then add 50mL dichloromethane solution, shake, separate liquid, extract the aqueous phase with dichloromethane three times, 30mL each time, combine the organic phase, wash twice with saturated brine, 10mL each time, and concentrate on a rotary evaporator to ...
Embodiment 3
[0047]
[0048] A kind of synthesis of N-nitro-1,2,3,4-tetrahydroquinoline:
[0049] ① Add 2.66g of 1,2,3,4-tetrahydroquinoline into a 50mL round bottom flask, add 25mL of acetonitrile, stir at room temperature to dissolve;
[0050] ② Slowly add 4.52 g of nitropyridinium salt to the reaction system in batches, and the ratio of the amount of substrate 1,2,3,4-tetrahydroquinoline to the nitropyridinium salt of the nitrating agent is 1: 1.3;
[0051] ③React directly in an air atmosphere, mix well at room temperature, slowly heat to 50°C, monitor the progress of the reaction with thin-layer chromatography, react at 0.1MPa for 7 hours, the raw materials are completely converted, stop the reaction, and cool to room temperature;
[0052] ④Add 50mL saturated NaHCO to the system 3 solution, then add 50mL dichloromethane solution, shake, separate liquid, extract the aqueous phase with dichloromethane three times, 30mL each time, combine the organic phase, wash twice with saturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com