Alpha-amylase gene and application thereof
A technology of amylase and gene, applied in genetically engineered bacteria and its application fields, can solve the problems of poor enzyme activity, instability, and insufficient purity of α-amylase, etc., and achieve simple culture medium and culture conditions, short cycle, and enzyme The effect of live stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: from bacillus subtilis ( Bacillus subtilis ) The nucleotide sequence of the amylase gene isolated in 48-1.
[0029] Genomic DNA of Bacillus subtilis was extracted by CTAB method, and 2 μl was used as a template for polymerase chain reaction (PCR). According to the conserved region of the published amylase homologous sequence, primers (primer F and primer R) were designed in combination with the vector used. , the primers, components and amplification conditions used in this PCR reaction process are as follows:
[0030]F: 5'-GGAATTCCATATGGTGAGAAGCAAAAAA-3' (SEQ ID NO.3)
[0031] R: 5'-CGGGATCCTTATTGTGCAGCTGCTTGTA-3' (SEQ ID NO.4)
[0032] The composition of the PCR amplification system is shown in Table 1.
[0033] 10×Taq Buffer
[0034] Amplification conditions: pre-denaturation at 94°C for 4min, denaturation at 94°C for 45S, annealing at 57°C for 45S, extension at 72°C for 90S, end filling at 72°C for 10min, three steps of denaturation, anne...
Embodiment 2
[0035] Embodiment 2: Construction of recombinant expression vector
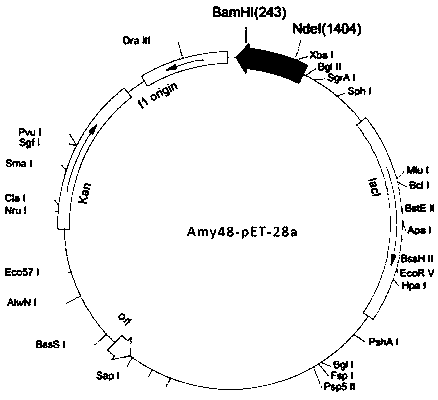
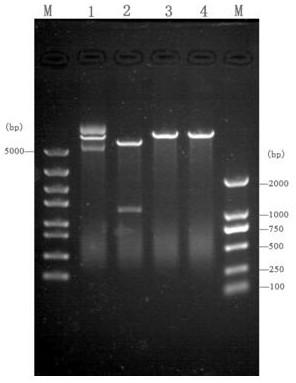
[0036] The fragment amplified in embodiment 1, because the 5 ' end of its primer F contains respectively Nde The I restriction site (from the 8th base to the 13th base) and the 5' end of the primer R contain Bam H I restriction site (3rd base to 8th base), so use these two enzymes to double-digest the fragment, and pET28a is double-digested with the same enzyme, and the large fragment is recovered by electrophoresis , and ligated with T4 ligase at 16°C for 6h, the schematic diagram of the successfully constructed vector is as follows figure 1 shown. The ligation product was transformed into Escherichia coli BL21(DE3) by chemical transformation method, cultured on LB solid plate containing kanamycin (100 μg / ml), and the white colonies growing on the plate were picked and analyzed by colony PCR ( figure 2 ) and extracted plasmid single and double digestion ( image 3 ) positive clones were screened and ...
Embodiment 3
[0037] Embodiment 3: the preparation of amylase
[0038] The recombinant engineered bacteria obtained in Example 2 was connected to 100ml LB liquid medium (containing 1‰ 50mg / ml kanamycin) according to 1% inoculation amount, and cultivated to OD at 37°C and 150rpm 600 =0.6, add 1‰ of 1mM IPTG (isopropyl-β-D-thiogalactopyranoside), turn to 16°C, induce 10h at 80rpm, collect the bacteria by centrifugation, and use 5ml 50mmol / l Suspended in imidazole buffer (pH 8.2) and sonicated. Take the supernatant by centrifugation, then suspend it with 5ml 50mmol / l imidazole buffer (pH8.2), take 50μl respectively for SDS polyacrylamide gel electrophoresis (SDS-PAGE), the band appears in the supernatant after centrifugation, Indicates that the obtained recombinant protein is not an inclusion body, such as Figure 4 Shown in lane 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com