Conjugated compound containing 9,9-diimidazolyl thiaxanthene and preparation and application thereof
A technology of conjugated compound and diimidazole, which is applied in the field of electroluminescent materials to achieve the effects of improving physical properties, good reproducibility and improving device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 2-bromophenylphenylsulfide (11mmol, 2.93g) in 60ml of anhydrous THF, cool to -78°C, add n-BuLi (14.36mmol) dropwise, keep warm for 1 hour, and mix N,N '-Carbonyldiimidazole (10mmol, 3.38g) was dissolved in 30ml THF and added dropwise to the reaction system overnight. Extract with dichloromethane, dry, and pass through a silica gel column to obtain a white solid. The obtained product was directly added to glacial acetic acid, heated to reflux, and 15 ml of concentrated hydrochloric acid was added to form a solid precipitate, which was filtered by suction to obtain the white solid product M1 (2.7 g, yield 50%). 1 HNMR: 7.43-7.45 (d, 2H), 7.37-7.40 (d, 4H), 7.17-7.23 (m, 6H), 6.87-6.90 (d, 4H), 6.62-6.65 (d, 2H).
[0030] (2) Dissolve M1 (2.7g, 7.7mmol) in 50mL of glacial acetic acid and heat to reflux. Add 2 times molar equivalent of liquid bromine and react overnight. It was extracted with dichloromethane, washed with water, dried, and the solvent was rem...
Embodiment 2
[0035] (1) M2 (1.5mmol, 0.81g), carbazole (3.3ml, 0.57g), CuI (0.23g), K 2 CO 3 (0.55g), C 18 o 6 (0.1g) was dissolved in DMPU, heated at 180°C overnight, extracted with dichloromethane, dried, extracted, and passed through the column to obtain 0.83g of conjugated compound P1 containing 9,9-diimidazolylthioxanthene, yield 82% . 1HNMR:8.33-8.34(d,2H),8.14-8.16(d,4H),7.65-7.67(d,4H),7.56-7.58(d,4H),5.49-5.51(d,4H),7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
[0036] (2) M3 (1.5mmol, 0.81g), carbazole (3.3ml, 0.57g), CuI (0.23g), K 2 CO 3 (0.55g), C 18 o 6 (0.1g) was dissolved in DMPU, heated at 180°C overnight, extracted with dichloromethane, dried, extracted, and passed through the column to obtain 0.83g of conjugated compound P2 containing 9,9-diimidazolylthioxanthene, yield 80% . 1 HNMR:8.85-8.84(d,2H),8.14-8.16(d,4H),7.65-7.67(d,4H),7.56-7.58(d,4H),5.49-5.51(d,4H),7.41-7.44 (d, 4H), 7.29-7.31 (m, 6H), 7.17-7.18 (d, 4H).
[0037] The reaction process ...
Embodiment 3
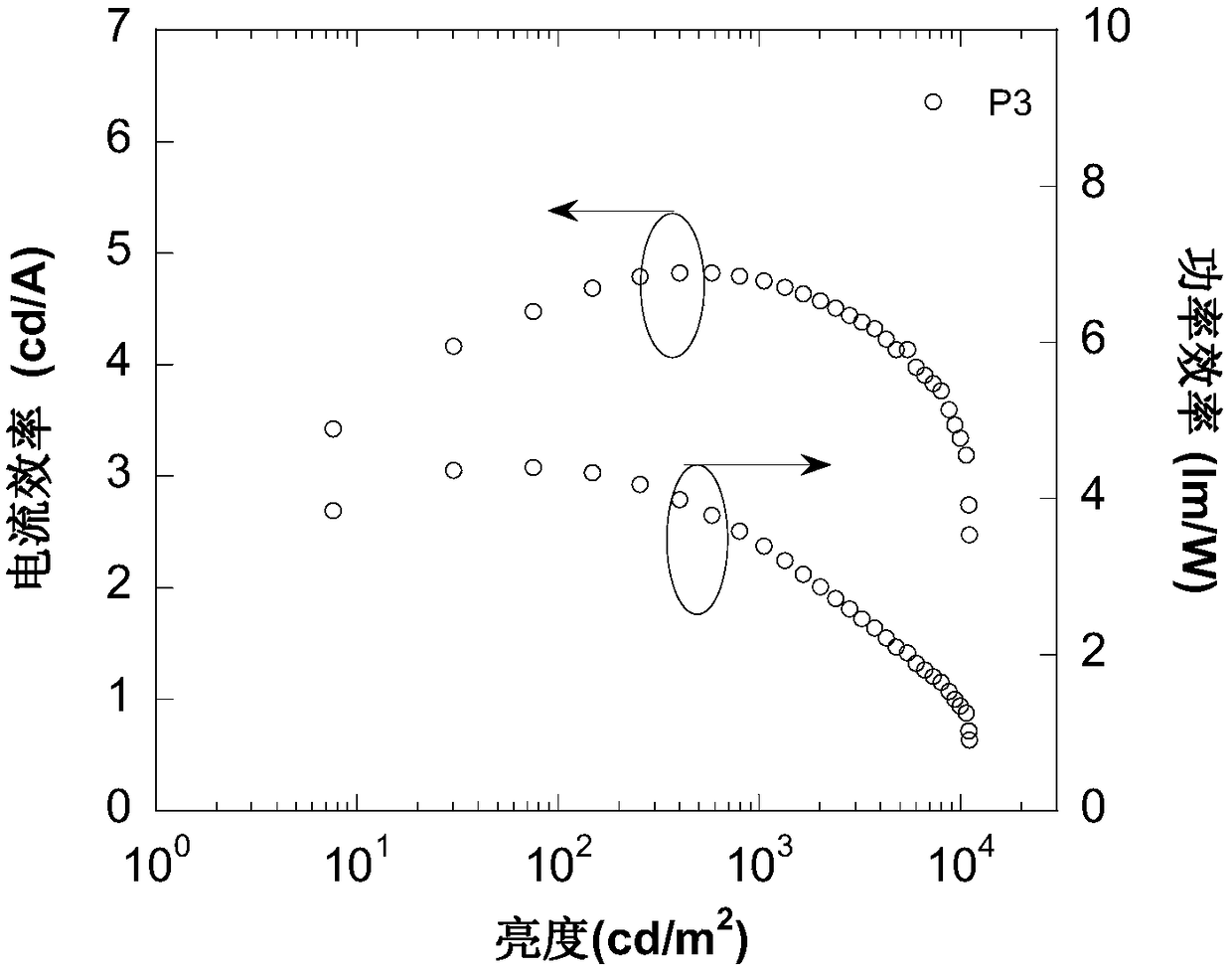
[0040] (1) M2 (1.5mmol, 0.81g), tert-butylcarbazole (3.3ml, 0.92g), CuI (0.23g), K 2 CO 3 (0.55g), C 18 o 6 (0.1g) was dissolved in DMPU, heated at 180°C overnight, extracted with dichloromethane, dried, extracted, and passed through the column to obtain 0.83g of conjugated compound P3 containing 9,9-diimidazolylthioxanthene, yield 82% .
[0041] (2) M3 (1.5mmol, 0.81g), carbazole (3.3ml, 0.57g), CuI (0.23g), K 2 CO 3 (0.55g), C 18 o 6 (0.1g) was dissolved in DMPU, heated at 180°C overnight, extracted with dichloromethane, dried, extracted, and passed through the column to obtain 0.83g of conjugated compound P4 containing 9,9-diimidazolylthioxanthene, yield 80% . 1 HNMR:8.85-8.84(d,2H),8.14-8.16(d,4H),7.65-7.67(d,4H),7.56-7.58(d,4H),5.49-5.51(d,4H),7.41-7.44 (d, 4H), 7.29-7.31 (m, 4H), 7.17-7.18 (d, 4H), 1.43 (d, 18H).
[0042] The reaction process of this embodiment is shown in the following formula:
[0043]
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com