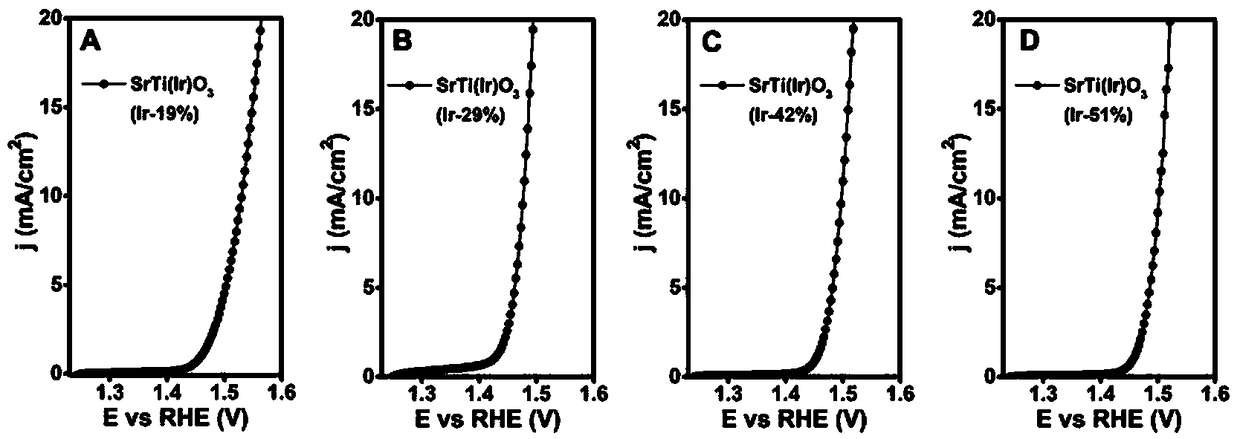
Iridium-based solid solution perovskite catalyst SrTi(Ir)O3 and application thereof in electrocatalytic water splitting for oxygen production
A solid solution, perovskite technology, applied in electrolytic components, electrolytic processes, electrodes, etc., can solve the problem of reducing the amount of Ir, and achieve the effects of reducing the amount of consumption, improving stability, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Iridium-Based Solid Solution Perovskite Catalyst SrTi(Ir)O 3 (Ir-19%) Preparation
[0034] Put 420mg (1.985mmol) of strontium nitrate, 280mg (1.332mmol) of citric acid and 80mg (0.166mmol) of potassium hexachloroiridate (IV) into 10mL of distilled water, stir well to form a dark brown transparent solution, called solution a; Transfer 225 mg (0.664 mmol) of tetrabutyl titanate into 4 mL of ethylene glycol, and stir well until a clear, colorless and transparent solution appears, which is called solution b. Slowly transfer solution a to solution b, heat and stir in a water bath at 70 degrees Celsius for 3 hours, and the solution appears brownish red transparent solution at this time. Subsequently, the solution was placed at 120° C. for 12 hours to ensure that the water was evaporated to dryness. In this example, n a :n b :( c +n d )=2.4:1.6:1; n c :n d =4:1. The evaporated solid sample was heated at 200°C, 300°C, 500°C and 600°C at a heating rate of 1.7°C / min for ...
Embodiment 2
[0047] Same as Example 1, only iridium-based solid solution perovskite SrTi(Ir)O 3 In the preparation of (Ir-19%), 420mg (1.985mmol) strontium nitrate was reduced to 350mg (1.654mmol), at this time n a :n b :(n c +n d )=2:1.6:1, the amount and conditions of other reactants remain unchanged, and what is obtained is still SrTi(Ir)O 3 (Ir-19%).
[0048] Iridium-Based Solid Solution Perovskite SrTi(Ir)O 3 In the preparation of (Ir-29%), 420mg (1.985mmol) strontium nitrate was reduced to 210mg (0.993mmol), at this time n a :n b :(n c +n d )=2:2.7:1, the amount and conditions of other reactants remain unchanged, and what is obtained is still SrTi(Ir)O 3 (Ir-29%).
[0049] Iridium-Based Solid Solution Perovskite SrTi(Ir)O 3 In the preparation of (Ir-42%), the amount of 420mg (1.985mmol) strontium nitrate is reduced to 140mg (0.661mmol), at this moment n a :n b :(n c +n d )=2:4:1, the amount and conditions of other reactants remain unchanged, and what is obtained is sti...
Embodiment 3
[0053] Same as Example 1, only iridium-based solid solution perovskite SrTi(Ir)O 3 In the preparation of (Ir-19%), 420mg (1.985mmol) strontium nitrate was increased to 3500mg (16.540mmol), at this time n a :n b :(n c +n d )=20:1.6:1, the amount and conditions of other reactants remain unchanged, and what is obtained is still SrTi(Ir)O 3 (Ir-19%).
[0054] Iridium-Based Solid Solution Perovskite SrTi(Ir)O 3 In the preparation of (Ir-29%), 420mg (1.985mmol) strontium nitrate was increased to 2100mg (9.925mmol), at this time n a :n b :(n c +n d )=20:2.7:1, the amount and condition of other reactants remain unchanged, and what is obtained is still SrTi(Ir)O 3 (Ir-29%).
[0055] Iridium-Based Solid Solution Perovskite SrTi(Ir)O 3 In the preparation of (Ir-42%), the amount of 420mg (1.985mmol) strontium nitrate is increased to 1400mg (6.620mmol), at this time n a :n b :(n c +n d )=20:4:1, the amount and conditions of other reactants remain unchanged, and what is obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com