P450BM3 mutant, and application of P450BM3 mutant in hydroquinone synthesis using benzene or phenol as substrate
A P450BM3, hydroquinone technology, applied in the application, plant genetic improvement, botanical equipment and methods, etc., can solve the problems of difficult hydroquinone yield, low hydroquinone yield, poor operation safety, etc. The effect of strong industrial application potential, short reaction time and good regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Site-directed mutagenesis of cytochrome P450-BM3 monooxygenase
[0043] The gene of wild-type cytochrome P450-BM3 monooxygenase (gene sequence shown in SEQ ID NO.2) was subjected to site-directed mutagenesis by using the large primer PCR mutagenesis technique.
[0044] Taking the 328th amino acid mutation as an example, first design a pair of primers for the mutation site.
[0045] The primers used were:
[0046] Upstream primer: 5'-CTTAATTGCGGGACACGAAACAACAAGTGGTC-3',
[0047] Downstream primer: 5'-CGCAGG aaa AGTTGGCCATAAGCGCAGC-3', where the underlined sequence is the mutation site.
[0048] The PCR system (20 μL) is: 0.5-20 ng template, 1 μL (10 μM) of each pair of mutant primers, 10 μL Prime STARMax DNA polymerase, and sterilized distilled water to make up to 20 μL.
[0049] The PCR reaction program is: (1) denaturation at 98°C for 3 min; (2) denaturation at 98°C for 10 sec, (3) annealing at 55°C for 15 sec, (4) extension at 72°C for 50 sec, and a to...
Embodiment 2
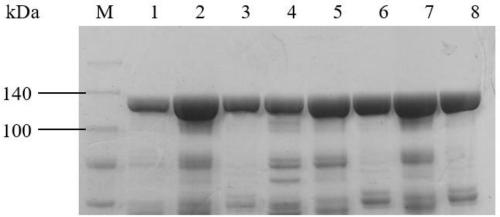
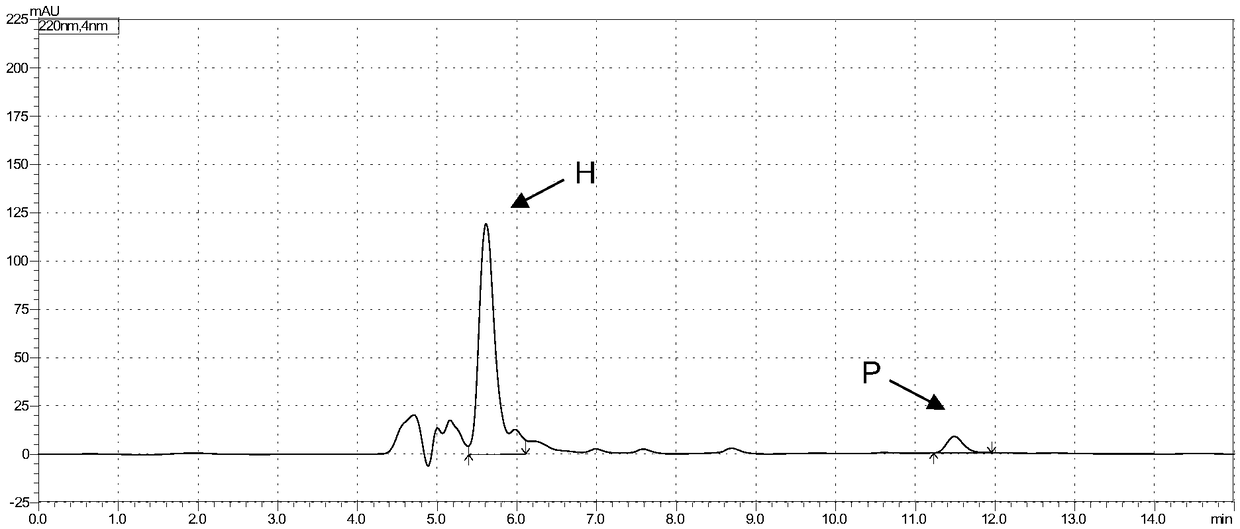
[0058] Example 2: Expression, purification and enzyme activity determination of cytochrome P450-BM3 monooxygenase mutants
[0059] The expression strain of the cytochrome P450-BM3 monooxygenase mutant obtained in Example 1 was inoculated in LB medium containing kanamycin (50 μg / mL) (peptone 10 g / L, yeast powder 5 g / L, NaCl 10 g / L), shake culture overnight at 37°C, take 500 μL of the bacterial solution and insert it into 200 mL of TB medium (peptone 12 g / L, yeast powder 24 g / L, glycerol 0.4%, KH 2 PO 4 2.31g / L, K 2 HPO 4 12.54g / L), placed in a 500mL Erlenmeyer flask at 37°C and 220rpm for shaking culture, when the absorbance of the culture solution OD 600 When it reaches 0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG) with a final concentration of 0.2mM for induction, and the induction temperature is 25°C. After induction for 14 to 20 hours, the culture medium is centrifuged , the cells were collected and washed twice with 0.1 M potassium phosphate buffer (pH 8.0) to o...
Embodiment 3
[0060] Example 3: Combinatorial Mutations of Cytochrome P450-BM3 Monooxygenases
[0061] On the basis of the mutation points V78F, A82F and A328F obtained by single-point saturation mutation, the three mutation sites of V78F, A82F and A328F were respectively combined to further obtain the combined mutants P450V78F / A82F, P450V78F / A328F, P450A82F / A328F and P450V78F / A82F / A328F.
[0062] Inoculate the recombinant Escherichia coli containing the combined mutation recombinant expression plasmid into LB medium (peptone 10g / L, yeast powder 5g / L, NaCl 10g / L) containing kanamycin (50μg / mL), and culture with shaking at 37°C Overnight, take 500 μL of bacterial solution and insert it into 200mL TB medium (peptone 12g / L, yeast powder 24g / L, glycerol 0.4%, KH 2 PO 4 2.31g / L, K 2 HPO4 12.54g / L), placed in a 500mL Erlenmeyer flask at 37°C and 220rpm for shaking culture, when the absorbance of the culture solution OD 600 When it reaches 0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG) wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com