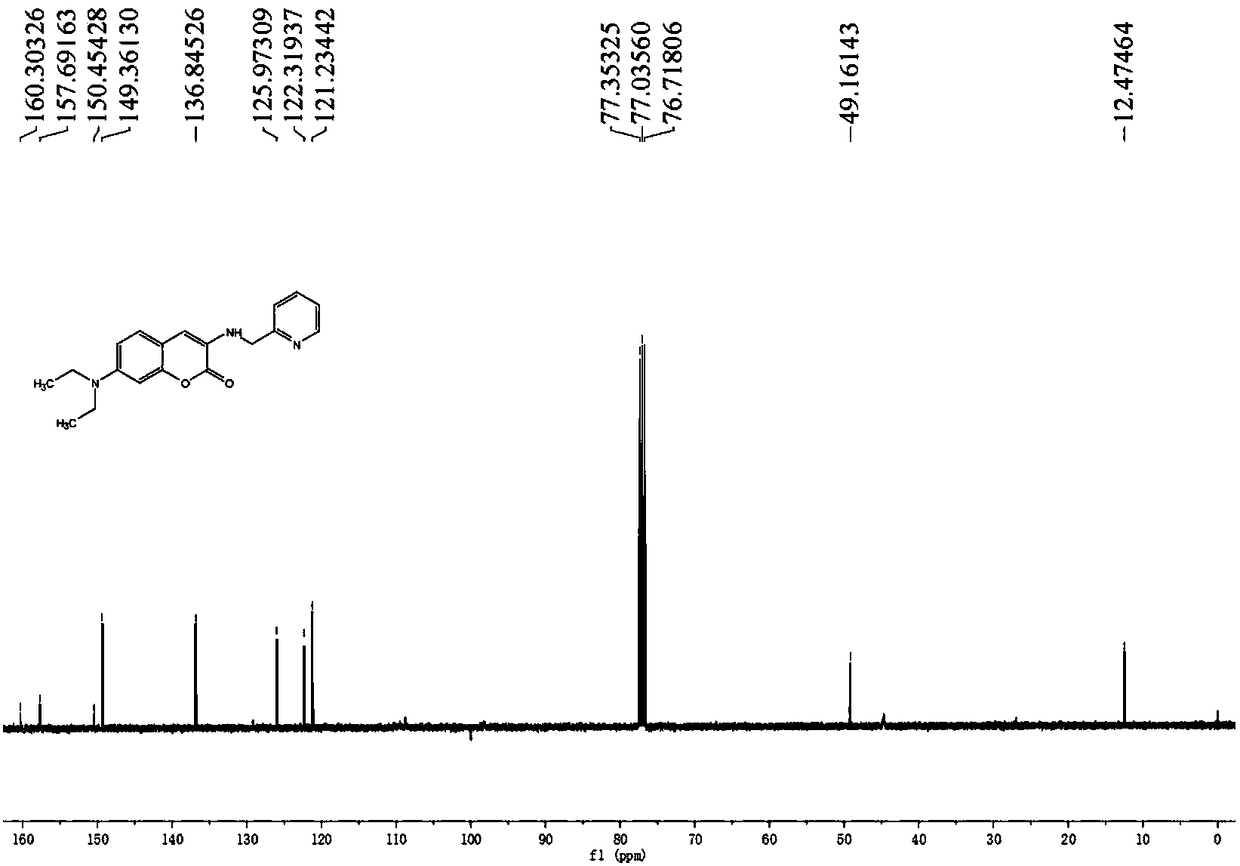
Pyridine methylene coumarin copper ion fluorescent probe and preparation thereof
A technology of coumarin and fluorescent molecular probes, which is applied in the field of coumarin derivative copper ion fluorescent probes and its preparation, can solve the problems of abnormal biological oxidation/reduction process and neurotoxicity, and achieve simple structure, High selectivity and sensitivity, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the synthesis of intermediate imine
[0021]
[0022] Add 1.16g of 3-amino-7-diethylaminocoumarin to 30mL of dichloromethane solution to dissolve, stir and add 618μL of pyridineformaldehyde under a magnetic stirrer, then add 2.13g of anhydrous sodium sulfate, and stir at room temperature for 24 hours . After the reaction was completed, filter, wash the solid sodium sulfate with a small amount of dichloromethane solution, and evaporate the solution to dryness under reduced pressure to obtain the crude product of the imine (the product was directly used in the next step without purification).
Embodiment 2
[0023] Embodiment 2: the synthesis of target compound I
[0024]
[0025] The crude product obtained in the previous step was dissolved in 30 mL of anhydrous methanol, and cooled in an ice bath. After the solution was completely cooled, 0.57 g of sodium borohydride was added in batches, and the reaction was continued for 2 hours in an ice bath, and the reaction was overnight at room temperature. After the reaction is over, evaporate the solution to dryness under reduced pressure, add 30 mL of distilled water and 30 mL of dichloromethane, stir and dissolve under a magnetic stirrer, pour the resulting aqueous solution directly into a separatory funnel, separate the liquids, and use 20 mL of dichloromethane for the water layer. After washing twice, the organic phases were combined, washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a viscous liquid. The obtained crude product was subjecte...
Embodiment 3
[0026] Embodiment 3: the mensuration of compound ultraviolet spectrum and fluorescence spectrum
[0027] Compound I was dissolved in DMSO to prepare a solution with a concentration of 2mM, and stored at 4°C; UV-Vis spectra and fluorescence spectra were in buffer solution (25mM HEPES, 0.1M NaClO 4 , pH=7.4, measured in 5% (V / V) DMSO). Add 2uL of fluorescent probe I solution into 2ml of buffer solution, mix well, and measure its ultraviolet-visible spectrum and fluorescence spectrum respectively. At the same time, the same amount of different metal ions were added to measure the change of its fluorescence properties.
[0028] The ultraviolet absorption spectrum of compound I is from as image 3 shown. Under simulated physiological environment (25mM HEPES, 0.1MNaClO 4 , pH=7.4, 5% (V / V) DMSO) before adding copper ions, the maximum absorption peak of compound I was at 226nm, and there were two absorption peaks at 265 and 389nm respectively. When copper ions are added to the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com