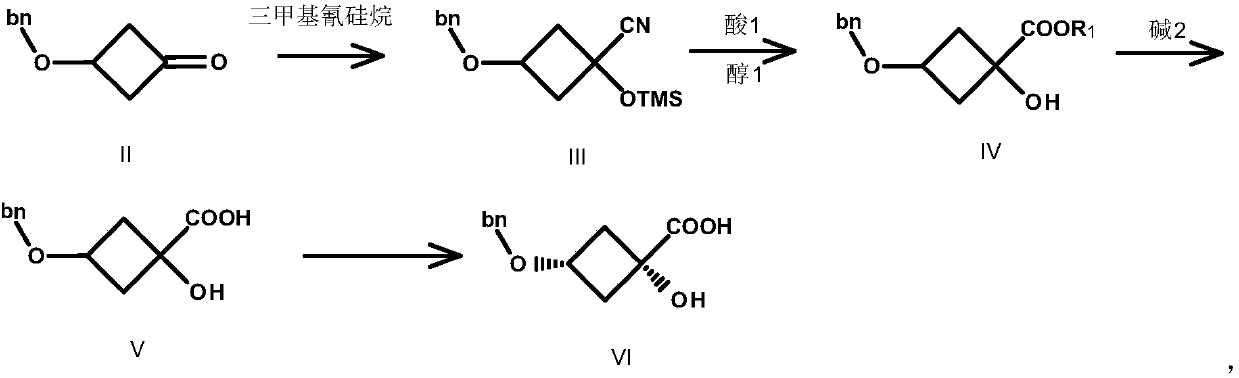
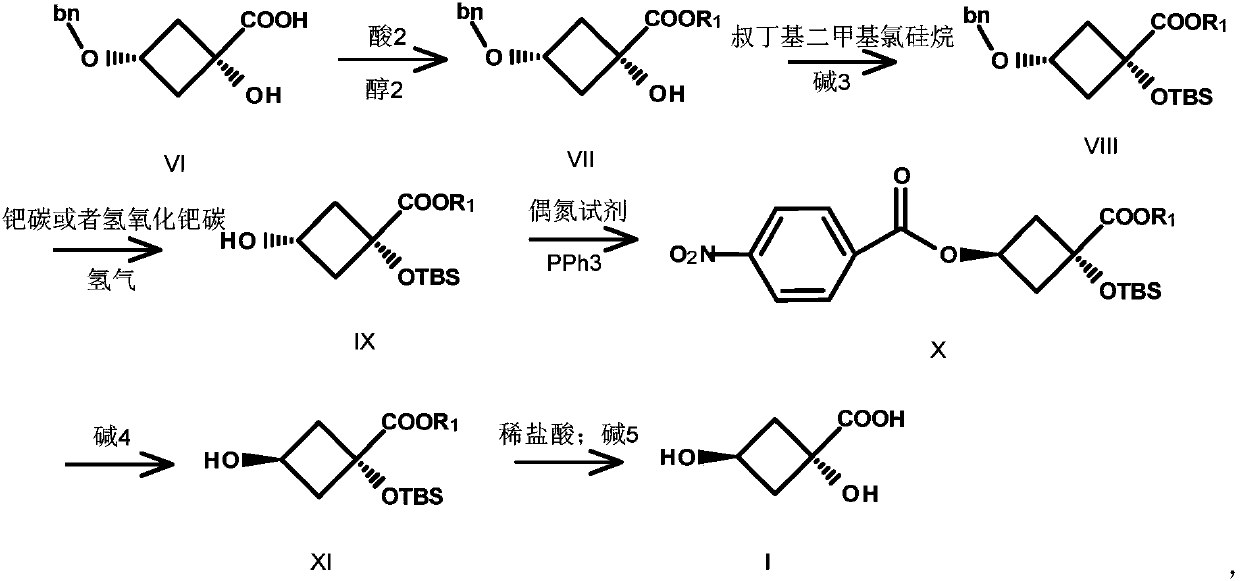
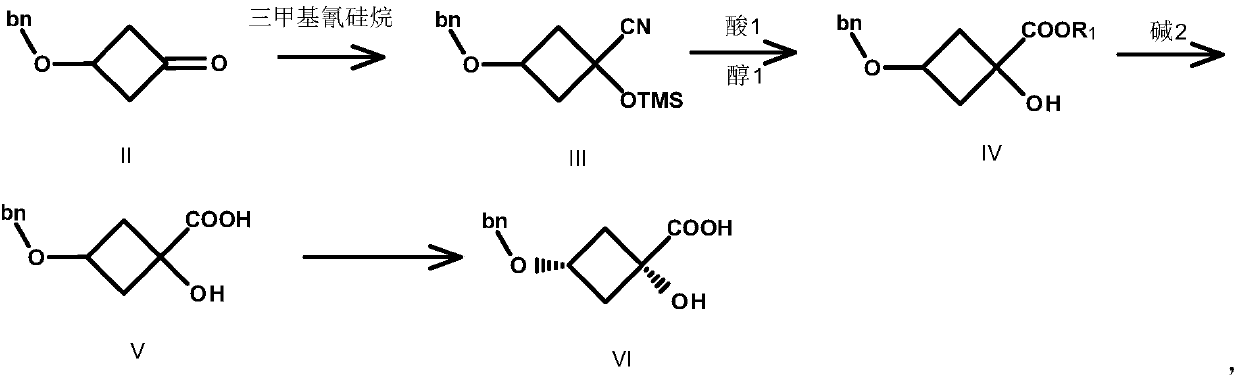
Preparation method of trans-1,3-dihydroxy-cyclobutane-1-carboxylic acid
A technology of trimethylsilyl cyanide and methyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of no literature reports on synthetic methods, and achieve the effects of rapid preparation, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Preparation of compound III
[0040] Compound II (80.00g, 0.454mol, 1.0eq.) was dissolved in 600mL THF, and K 2 CO 3 (75.32g, 0.545mol, 1.2eq.) and TMSCN (54.05g, 0.545mol, 1.2eq.), reacted at 20°C for 3h, TLC detected that the reaction was complete, filtered, and the filtrate was concentrated to obtain compound III as a red liquid 120.0g, yield : 96.0%. (ESI-TOF) m / z: [M+H] + calcd for C 15 h 21 NO 2 Si: 275; found: 276.
[0041] Preparation of Compound IV-1
[0042] Compound III (104.11g, 0.378mol, 1.0eq.) was dissolved in 500mL MeOH, and SOCl was added dropwise under ice-cooling 2 (80.90g, 0.684mol, 1.8eq.), the temperature was controlled below 35°C, after dropping, heated to reflux at 55°C for 3h, a large amount of solids were formed. TLC detects that the reaction is complete. Methanol was concentrated, 500 mL of tertiary methyl ether was added, filtered, the filtrate was washed with water, dried, and concentrated to obtain 80.0 g of compound I...
Embodiment 2
[0060]
[0061] Preparation of Compound III
[0062] Compound II (80.00g, 0.454mol, 1.0eq.) was dissolved in 600mL THF, and Na 2 CO 3 (24.06g, 0.227mol, 0.5eq.) and TMSCN (45.04g, 0.454mol, 1.0eq.), reacted at 20°C for 3h, TLC detected that the reaction was complete, filtered, and the filtrate was concentrated to obtain compound III as a red liquid 112.5g, yield : 90.0%. (ESI-TOF) m / z: [M+H] + calcd for C 15 h 21 NO 2 Si: 275; found: 276.
[0063] Preparation of Compound IV-2
[0064] Compound III (100.1g, 0.363mol, 1.0eq.) was dissolved in 500mL EtOH, and concentrated sulfuric acid (3.56g, 0.0363mol, 0.1eq.) was added dropwise under ice-cooling. Refluxing at 55°C for 4h, a large amount of solids were formed. TLC detects that the reaction is complete. Concentrate methanol, add 500 mL of tertiary methyl ether, filter, wash the filtrate with water, dry, and concentrate to obtain 78.3 g of compound IV-2 as a brown liquid, yield: 87%. (ESI-TOF) m / z: [M+H] + calcd fo...
Embodiment 3
[0082]
[0083] Preparation of compound III
[0084] Compound II (80.00g, 0.454mol, 1.0eq.) was dissolved in 1000mL of acetonitrile, cesium carbonate (295.8g, 0.908mol, 2.0eq.) and TMSCN (90.07g, 0.908mol, 2.0eq.) were added, at 20°C After reacting for 3 hours, TLC detected that the reaction was complete, filtered, and the filtrate was concentrated to obtain 112.5 g of compound III as a red liquid, with a yield of 90.0%. (ESI-TOF) m / z: [M+H] + calcd for C 15 h 21 NO 2 Si: 275; found: 276.
[0085] Preparation of Compound IV-1
[0086] Compound III (102.1g, 0.370mol, 1.0eq.) was dissolved in 500mL of methanol, and SOCl was added dropwise under ice-cooling 2 (132.32g, 1.11mol, 3.0eq.), the temperature was controlled below 35°C, after dropping, heated to reflux at 55°C for 5h, a large amount of solids were formed. TLC detects that the reaction is complete. Concentrate methanol, add 500 mL of tertiary methyl ether, filter, wash the filtrate with water, dry, and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com