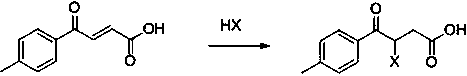Preparation method of zolpidic acid
A technology of zolpidem acid and butyric acid, which is applied in the field of preparation of pharmaceutical intermediate zolpidem acid, can solve the problems of explosives, inconvenient storage and transportation, and high toxicity, and achieve easy operation, simple process steps, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1) Add 9.8g of maleic anhydride, 9.2g of toluene and 200ml of dichloromethane into a 1L three-necked flask, cool down to 0°C in a low-temperature bath under mechanical stirring, and then slowly add 27g of aluminum trichloride into the reaction flask to maintain the temperature 0-10°C, after adding, stir at room temperature and react for more than 4 hours. HPLC detects that the reaction is complete. Pour the reaction solution into 500g of ice water to quench, then extract with 500g of ethyl acetate, remove the solvent, and dry to obtain 16.2g of yellow solid. mp. 120.8-123.1°C; 1H-NMR (400 MHz, CDCl3)δ 8.00 (d, J=15.5 Hz, 1H), 7.92 (d, J=8.1 Hz, 2H),7.33 (d, J=8.1 Hz , 2H), 6.89 (d, J=15.5 Hz, 1H), 2.45 (s, 3H), yield 85.0%.
[0036] Step 2) Add 19.0g of 4-oxo-4-(4-methylphenyl)-2-butenoic acid, 20.2g of 48% hydrobromic acid and 500g of dichloroethane into a 1000mL reaction flask, and heat up to reflux for reaction. After the addition reaction was completed, let it...
Embodiment 2
[0039] Step 1) Add 19.6g maleic anhydride and 200g toluene to a 1L three-necked flask, cool down to 0°C in a low-temperature bath under mechanical stirring, then slowly add 54g of aluminum trichloride to the reaction flask, keep the temperature at 0-10°C, add After completion, the temperature was raised to room temperature and the reaction was stirred for more than 4 hours. HPLC detected that the reaction was complete. The reaction solution was poured into 500 g of ice water to quench, then extracted with 500 g of ethyl acetate, the solvent was removed, and dried to obtain 30.0 g of a yellow solid, mp. 121.3- 123.2°C; 1H-NMR (400 MHz, CDCl3) δ 8.00(d, J=15.5 Hz, 1H), 7.92 (d, J=8.1 Hz, 2H),7.33 (d, J=8.1 Hz, 2H), 6.89 (d, J=15.5 Hz, 1H), 2.45 (s, 3H), yield 78.7%.
[0040] Step 2) Add 19.0g of 4-oxo-4-(4-methylphenyl)-2-butenoic acid, 18.3g of 20% hydrogen chloride methanol solution and 500g of toluene into a 1000mL reaction flask, heat up and reflux for reaction, after the re...
Embodiment 3
[0043] Step 1) Add 19.6g of maleic anhydride, 18.4g of toluene and 200ml of dichloroethane into a 1L three-necked flask, cool down to 0°C in a low-temperature bath under mechanical stirring, and then slowly add 27g of aluminum trichloride into the reaction flask, keeping Temperature 0-10°C, after addition, stir at room temperature for more than 4 hours, HPLC detects that the reaction is complete, pour the reaction solution into 500g of ice water to quench, then extract with 500g of ethyl acetate, remove the solvent, and dry to obtain 32.4g of yellow solid , mp. 120.8-123.1°C; 1H-NMR (400 MHz, CDCl3) δ 8.00 (d, J=15.5 Hz, 1H), 7.92 (d, J=8.1 Hz, 2H),7.33 (d, J=8.1 Hz,2H), 6.89 (d, J=15.5 Hz, 1H), 2.45 (s, 3H), yield 85.0%.
[0044] Step 2) Add 19.0g of 4-oxo-4-(4-methylphenyl)-2-butenoic acid, 27.9g of 50% hydroiodic acid solution and 200g of xylene into a 1000mL reaction flask, and raise the temperature at 110-120 ℃ reaction, after the addition reaction is completed, let stan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com