Preparation of 1,2-diaryl benzimidazole derivative and application thereof
A benzimidazole and diaryl technology, applied in the field of medicinal chemistry, can solve the problems of poor water solubility, poor structural stability, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
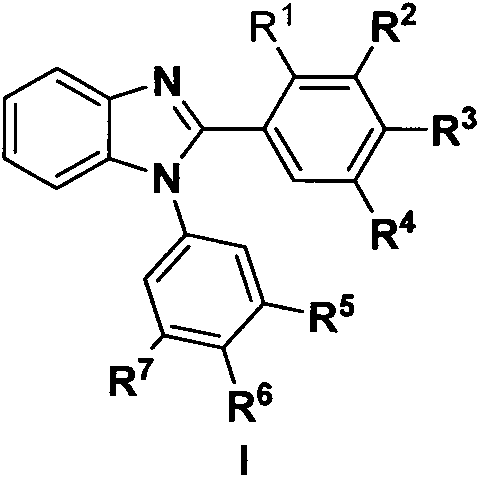
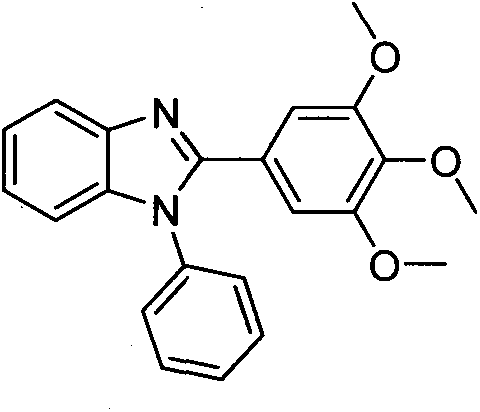
[0019] Preparation of 1-phenyl-2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (4a)
[0020]
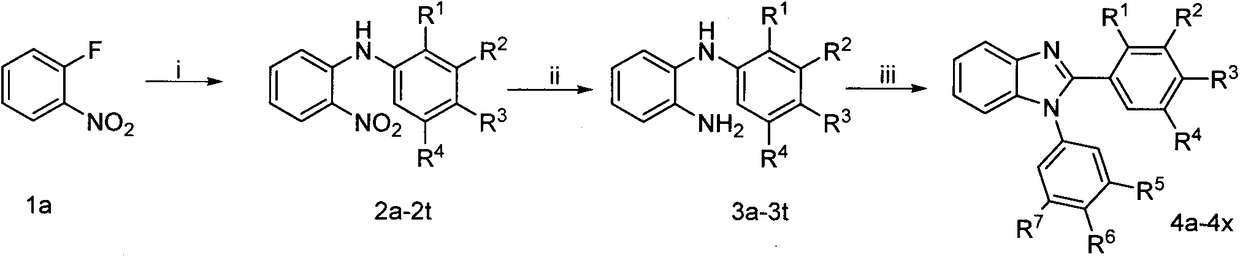
[0021] Add o-fluoronitrobenzene (1.41g, 10mmol) and 3,4,5-trimethoxyaniline (1.692g, 12mmol) into water (20mL), stir and reflux for 6h. The reaction solution was cooled to room temperature and washed with NaHCO 3 (about 1.7g) neutralized, and extracted with ethyl acetate (3×100mL), the combined ethyl acetate extracts were washed with water, dried over anhydrous sodium sulfate, and evaporated to dryness in vacuo to obtain the crude compound 2a, which was purified by silica gel column chromatography ( Eluent system: petroleum ether / ethyl acetate) to obtain compound 2a as orange powder. Under ice-bath conditions, a mixed solution containing compound 2a (6.37g, 20.0mmol) in tetrahydrofuran (100mL) and absolute ethanol (50mL) was added dropwise to a solution of sodium dithionite (55.7g, 320mmol) in water (125mL) , and then the mixed solution was stirred at room temperature for 1 h, a...
Embodiment 2
[0023] Preparation of 2-methoxy-5-(2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazol-1-yl)phenol (4b)
[0024]
[0025] The preparation method refers to Example 1. A white powder was obtained, yield: 65.6%, m.p.194-196°C. 1 H NMR (600MHz, DMSO-d6) δ7.83(s, 1H), 7.26(d, J=12.9Hz, 1H), 7.09(d, J=10.5Hz, 1H), 6.73(t, J=15.4Hz , 1H), 6.51-6.44(m, 2H), 6.37(d, J=5.5Hz, 2H), 6.29-6.22(m, 2H), 3.83(s, 3H), 3.71-3.64(m, 6H), 3.53(s, 3H).
Embodiment 3
[0027] Preparation of 1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (4c)
[0028]
[0029] The preparation method refers to Example 1. A white powder was obtained, yield: 77.5%, m.p.171-173°C. 1 H NMR (600MHz, DMSO-d6) δ7.02(d, J=18.6Hz, 1H), 6.83(t, J=12.0Hz, 1H), 6.71-6.67(m, 3H), 6.52(s, 1H) , 6.28(m, 2H), 6.17(s, 2H), 3.91(s, 3H), 3.83(s, 3H), 3.75(s, 3H), 3.67(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com