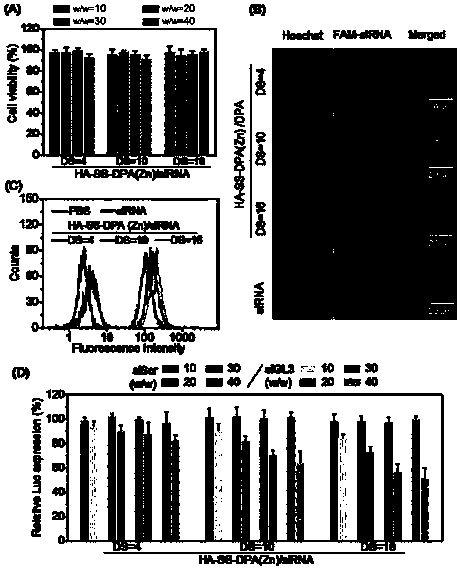
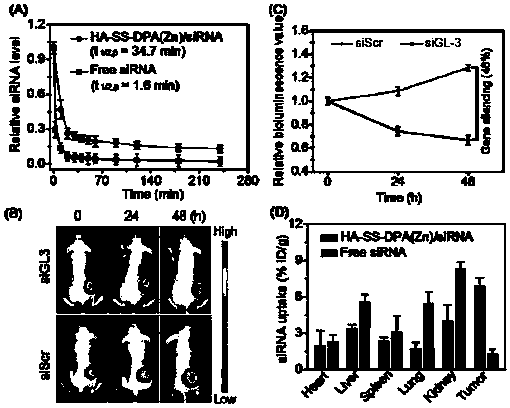
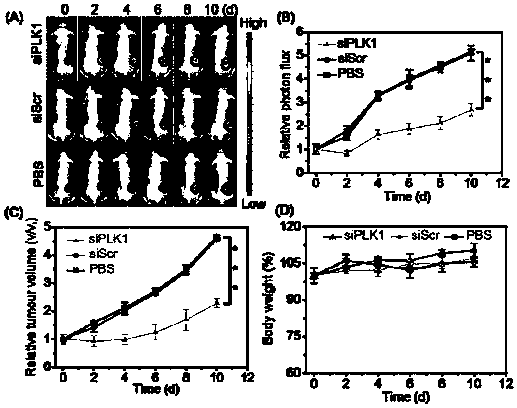
Nano-drug and synthesis method thereof
A synthesis method and nano-drug technology, applied in the direction of drug combination, medical formula, genetic material components, etc., can solve the problems of cell membrane instability, non-cancerous tissue cytotoxicity, etc., achieve good therapeutic effect, avoid normal tissue lesions, and prolong half-life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The synthetic method of nano medicine, comprises the steps:
[0041] (1) Synthesis of DxDPA monomer
[0042] 2,2'-dipicolylamine reacted with α,α'-dichloro-p-xylene to obtain DxDPA monomer. Specifically, both 2,2'-dipicolylamine and α,α'-dichloro-p-xylene were dissolved in methyl chloride and reacted for 24-28 hours under inert gas conditions to obtain DxDPA monomer.
[0043] Optionally, the molar ratio of 2,2'-dipicolylamine to α,α'-dichloro-p-xylene is 1:(1.5-2.5).
[0044] The specific steps are as follows: 2,2'-dipicolylamine (DPA) (8mmol) and α,α'-dichloro-p-xylene (Dx) (16mmol) were dissolved in dichloromethane or chloroform, and then added Anhydrous K 2 CO 3 (40mmol) or anhydrous sodium sulfate to remove water. And stirred at room temperature and inert gas (nitrogen as an option) for 24 hours to obtain DxDPA monomer.
[0045]
[0046] Since the DxDPA monomer solution contains certain impurities such as reactants and organic solvents, the sample is extrac...
Embodiment 1
[0067] The synthetic method of nano medicine, comprises the steps:
[0068] (1) Synthesis of DxDPA monomer: 2,2'-dipicolylamine reacts with α,α'-dichloro-p-xylene to obtain DxDPA monomer.
[0069] (2) Synthesis of DxDPA-Cys polymer: react cystamine dihydrochloride and triethylamine to obtain cystamine, then add monomer to obtain DxDPA-Cys polymer.
[0070] (3) Synthesis of HA-SS-DPA polymer: react hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide Then add the mixed solution of triethylamine and DxDPA-Cys polymer and react to obtain HA-SS-DPA polymer.
[0071] (4) Synthesis of HA-SS-DPA(Zn) / siRNA nanomedicine: Dissolve HA-SS-DPA polymer in buffer solution and add chelating agent to react to obtain HA-SS-DPA(Zn), and HA-SS- DPA(Zn) is mixed with small fragments of interfering RNA and incubated under the condition of 55-65°C to obtain nano-medicine.
Embodiment 2
[0073] The synthetic method of nano medicine, comprises the steps:
[0074] (1) Synthesis of DxDPA monomer: 2,2'-dipicolylamine and α,α'-dichloro-p-xylene with a molar ratio of 1:1.5 or 1:2.5 or 1:2 are dissolved in chlorine DxDPA monomer was obtained by reacting in methane for 24h or 26h or 28h under the condition of inert gas.
[0075] (2) Synthesis of DxDPA-Cys polymer: First, dissolve cystamine dihydrochloride and triethylamine in dimethyl sulfoxide with a molar ratio of 1:2 or 1:4 or 1:3 in an inert gas Under the conditions of reaction for more than 1.5h to obtain the first mixed solution, then dissolve the DxDPA monomer in dimethyl sulfoxide and add it to the first mixed solution, and react at 55°C or 60°C or 65°C for 12h or 14h or DxDPA-Cys polymer was obtained in 16h; wherein, the molar ratio of cystamine dihydrochloride to DxDPA monomer was 1:0.2 or 1:0.3 or 1:0.4.
[0076] (3) Synthesis of HA-SS-DPA polymer: hyaluronic acid with a molar ratio of 1:4:4 or 1:5:5 or 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com