A targeting material based on indole squaraine dye and its preparation method, fluorescent nanoparticles and its preparation method
A technology of indole squaraine and fluorescent nanotechnology, applied in the field of biomedicine, can solve the problems of weak application in the field of cancer photodiagnosis and treatment, lack of specific tumor targeting ability and cancer treatment ability, and achieve good product stability and biological High safety and high tumor suppression rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
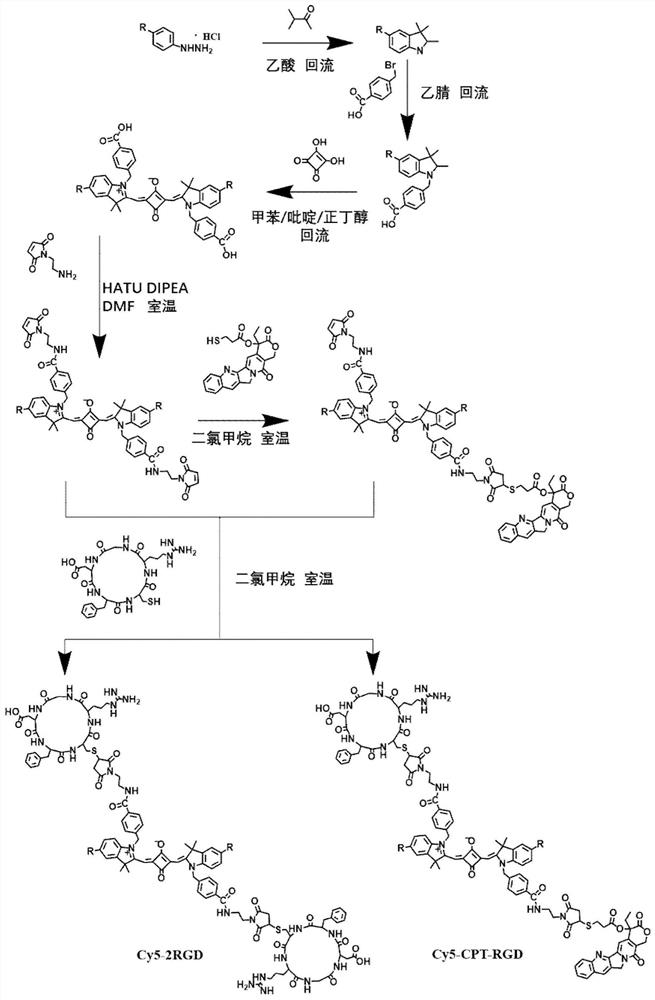
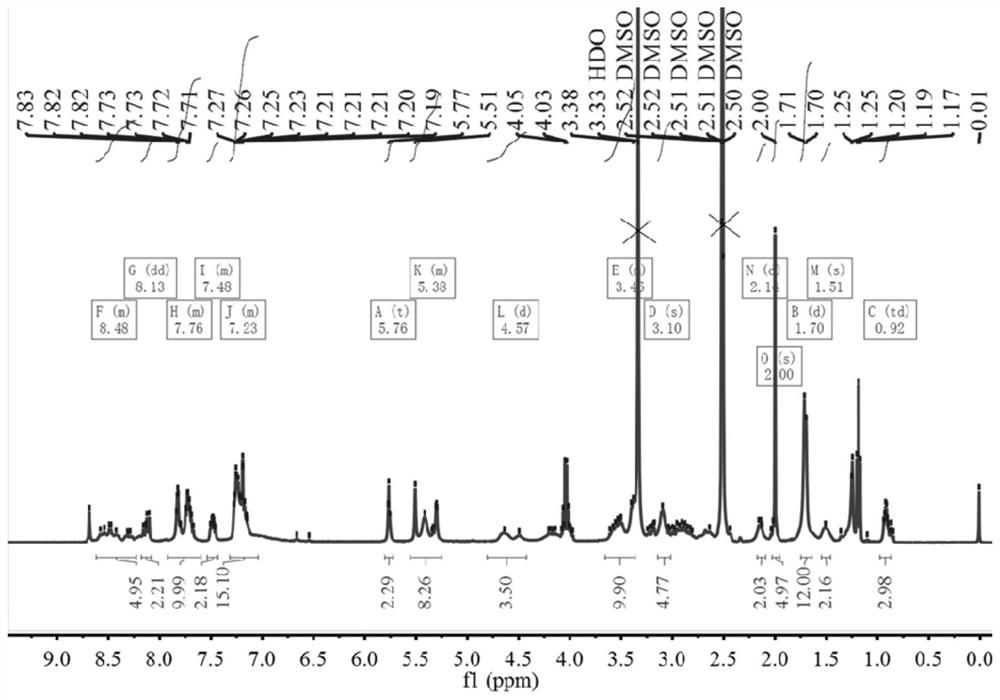
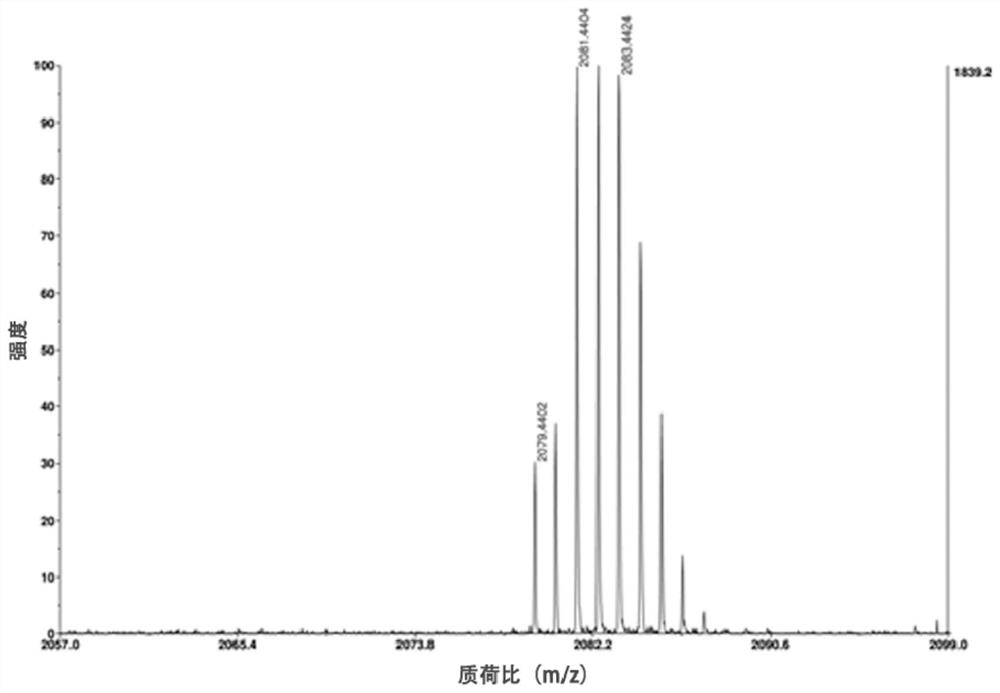
[0042] Example 1: Synthesis of indole squaraine dye ICy5-CPT-RGD for targeted diagnosis and treatment
[0043] 1. Add 2.23g (10mmol) of 4-bromophenylhydrazine hydrochloride and 0.86g (10mmol) of 3-methyl-2-butanone into 10mL of glacial acetic acid, reflux for 12 hours, and evaporate to remove the solvent. Dissolve with dichloromethane and wash with saturated NaHCO3 The aqueous solution was washed repeatedly until the aqueous phase was colorless and the pH was neutral, and the organic phase was removed by rotary evaporation to obtain 1.88 g (7.9 mmol) of 5-bromo-2,3,3-trimethyl-3H-ylindole with a yield of 79%. ;
[0044] 1 H NMR (400MHz, CDCl 3 )δ7.40(s,3H),2.27(s,3H),1.30(s,6H).
[0045] 2. Add 1.19g (5mmol) of 5-bromo-2,3,3-trimethyl-3H-ylindole and 1.08g (5mmol) of p-benyl bromobenzoic acid to 15mL of acetonitrile, reflux for 20 hours, and remove by rotary evaporation After the solvent was precipitated with ether and washed several times, 1.72 g (3.8 mmol) of the interme...
Embodiment 2
[0055] Example 2: ICy5-CPT-RGD prepares nanoparticle PTN by self-assembly method
[0056] Dissolve 2.1 mg of indole squaraine dye ICy5-CPT-RGD in 1 mL of DMSO (1×10 -3 M), 0.1 mL of the dye solution was dropped into 10 mL of water (50° C.), stirred rapidly for 5-10 minutes, and then DMSO was removed by dialysis to obtain an aqueous solution of PTN.
Embodiment 3
[0057] Example 3: PTN nanoparticles for targeted fluorescence imaging of living tumors
[0058] A tumor model of human liver cancer in vitro (BEL-7402) in Balb / c nude mice was established to evaluate the targeting imaging ability and anti-tumor effect of PTN in vivo. The cultured BEL-7402 cells were mixed with 3×10 6 Cells / 100 μL were inoculated subcutaneously at the lower right of the back of nude mice, and cultured until the tumor volume (0.5 × length × width 2 ) is 100mm 3 about. The tumor-bearing mice were injected with 200 μL of PTN (1 mg / mL) solution through the tail vein, and the control group was injected with 200 μL of normal saline. The mice were placed in an IVIS light-shielding incubator, then the mice were anesthetized with 2.5% isoflurane, and images were collected using the IVIS small animal in vivo imaging system (PerkinElmer Co.). The time points for collecting images were 0, 0.5, 1, 3, 6, 12, 18, and 24 hours. After 24 hours, the mice were sacrificed, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com