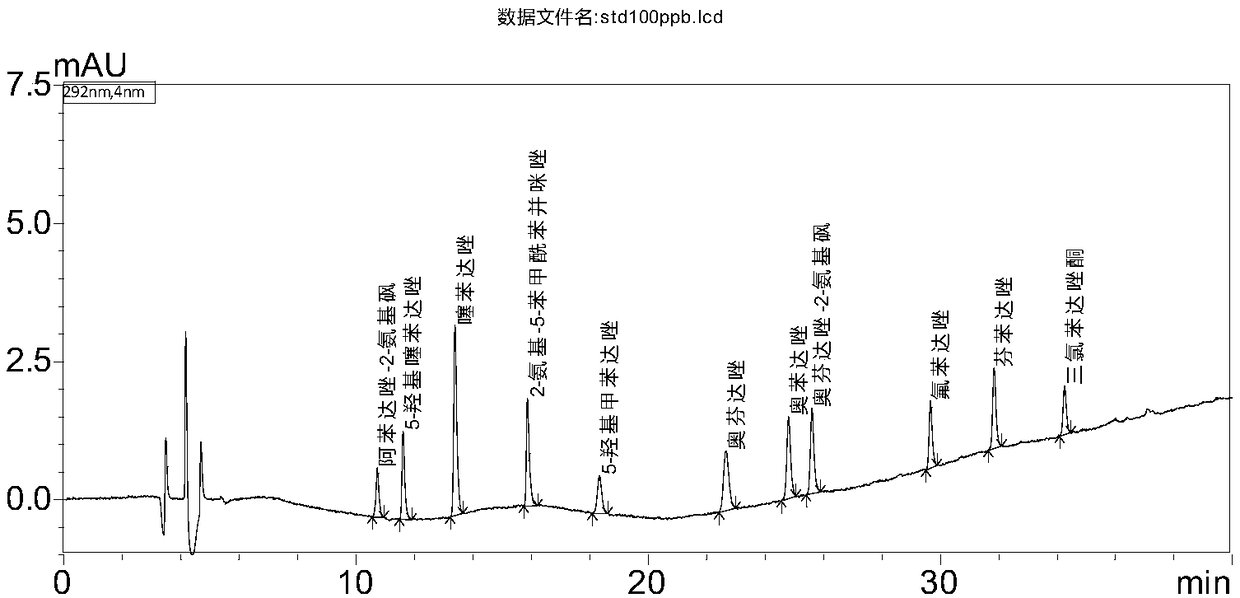
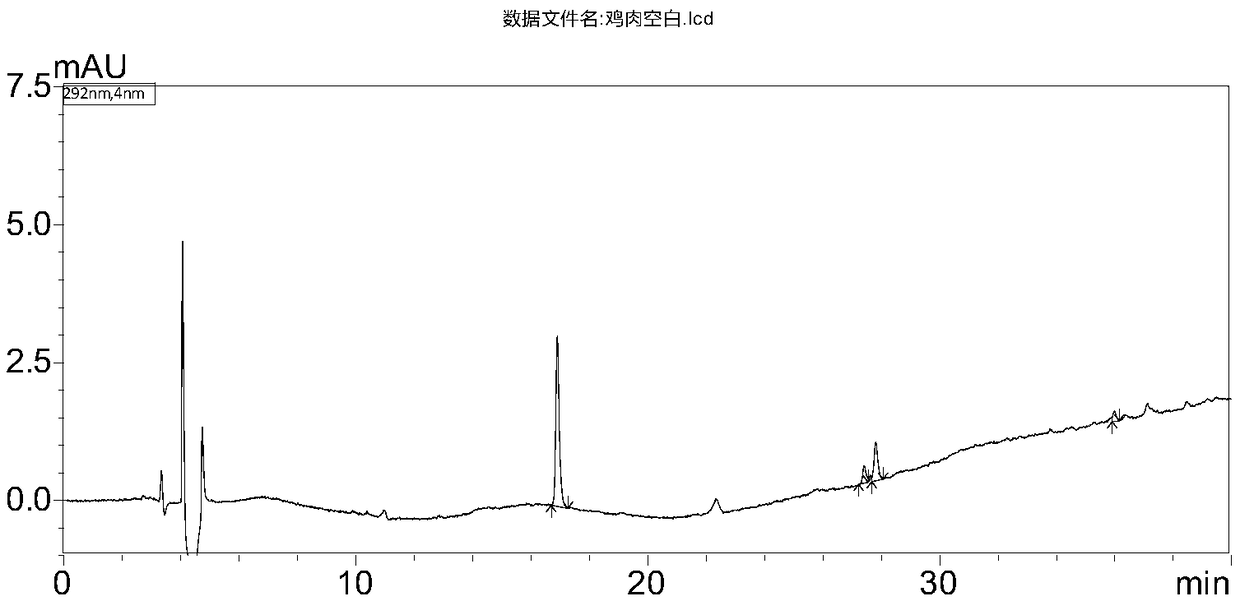
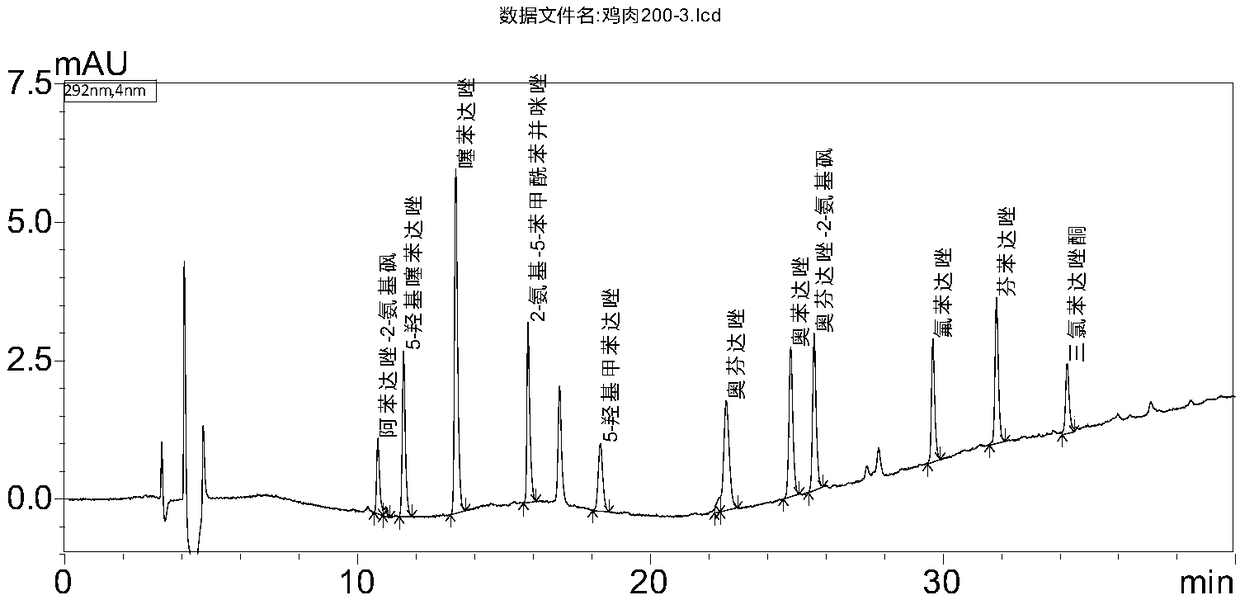
Determination method of benzimidazoles drug residues in chicken tissues
The technology of a benzimidazole and a determination method is applied in the field of determination of the residual amount of benzimidazoles in chicken tissue, and can solve problems such as technical difficulties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Establishment method
[0037] 1. Reagents and Materials
[0038] The reagents used below are all analytical reagents unless otherwise specified; the water is first-class water in accordance with GB / T 6682.
[0039] Standard: Oxfendazole (CAS No.: 53716-50-0), Fenbendazole (CAS No.: 43210-67-9), Oxfendazole-2-aminosulfone (Oxfendazole sulphone, CAS No.: 54029-20-8), Albendazole-2-aminosulfone (Albendazole-2-aminosulfone, CAS No.: 80983-34-2), Thiabendazole (CAS No.: 148-79- 8), 5-hydroxythiabendazole (5-hydroxythiabendazole, CAS number: 948-71-0), 2-amino-5-benzoylbenzimidazole (Mebendazole-amine, CAS number: 52329-60-9 ), 5-Hydroxymebendazole (5-Hydroxymebendazole, CAS No.: 60254-95-7), Flubendazole (Flubendazole, CAS No. 31430-15-6), Oxibendazole (Oxibendazole, CAS No.: 20559 -55-1) and triclabendazole (Ketotriclabendazole, CAS No.: 1201920-88-8), the content of which is above 97%.
[0040] Acetonitrile: chromatographically pure; methanol: chromatographi...
Embodiment 2
[0111] Embodiment 2: the selection of extraction solvent
[0112] The present invention compares acetonitrile and the basic ethyl acetate used in GB / T 21324-2007 "Methods for the Detection of Benzimidazole Drug Residues in Edible Animal Muscle and Liver". Under the chromatographic system conditions of the method, acetonitrile extracts The interference peaks of the liquid are far less than that of basic ethyl acetate. In addition, the basic ethyl acetate extract must be converted to solvent before being purified and put on the column in the next step. 0.1mol / L hydrochloric acid solution is dissolved, and the concentration process will cause the degradation of triclabendazolone, while the acetonitrile extract does not need to be concentrated, and can be loaded on the column after adding 0.1mol / L hydrochloric acid solution. The extraction efficiency of all the compounds in the acetonitrile extraction liquid reaches more than 95%, so the present invention selects acetonitrile as t...
Embodiment 3
[0113] Embodiment 3: the selection of purification conditions
[0114] At first, the present invention tries to adopt reverse-phase adsorbent HLB solid-phase extraction small column, when the acetonitrile ratio in the upper column solution is 10%, oxfendazole-2-aminosulfone and 5-hydroxythiabendazole will be lost to some extent, Therefore, the acetonitrile extract must be concentrated, which will not only cause the degradation of the compound to be tested, but also cause the proportion of organic solvent to be too low during re-dissolution, resulting in poor dissolution effect and lower recovery rate.
[0115] After comparison, this method adopts MCX solid-phase extraction small column, for triclabendazolone, the N-vinylpyrrolidone-divinylbenzene copolymer in the filler has played the effect of reverse phase adsorbent; As far as the compound is concerned, the N-vinylpyrrolidone-divinylbenzene copolymer matrix-SO3H in the filler mainly plays the role of ion exchange. Combining...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com