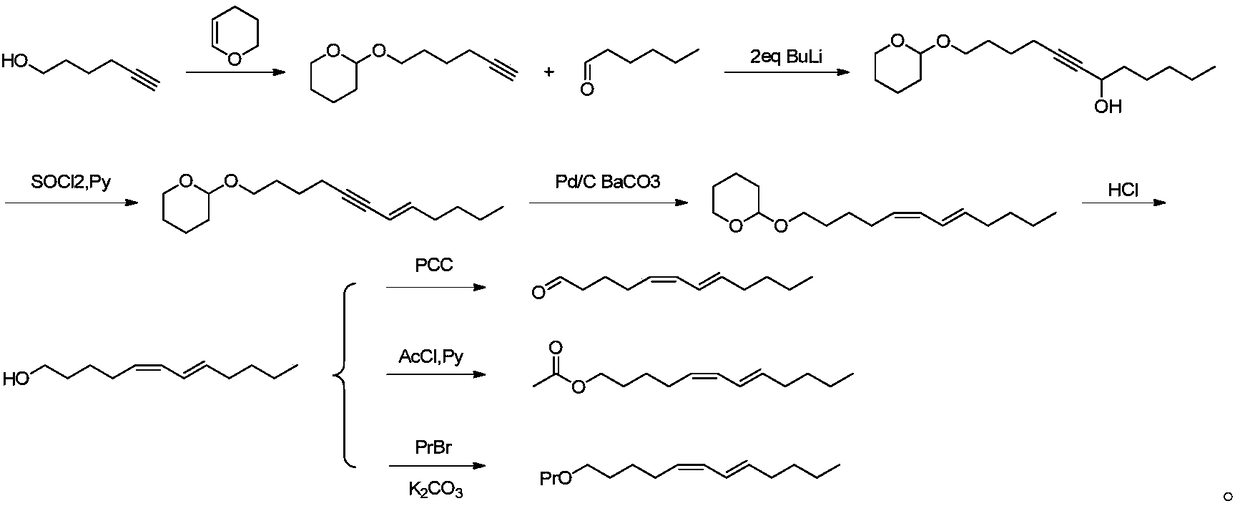
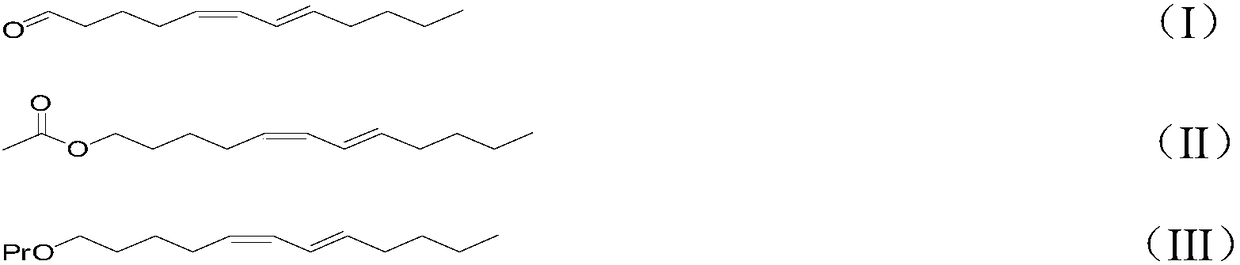Synthetic method for attractant component namely cis,trans-5,7-dodecadiene derivative in Dendrolimus sex pheromone
A synthesis method and pheromone technology, which are applied in attracting pests, botany equipment and methods, chemicals for biological control, etc., can solve the problems of low yield, high cost, unfavorable application, etc., and achieve low price, The effect of short reaction steps and ease of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 5-hexyn-1-alcohol (11.8g, 0.12mol) and 3,4-dihydro-2H-pyran (8.4g, 0.1mol) in reaction flask, then add 1g solid acid catalyst phosphotungstic acid ( h 3 PW 12 o 40 ), 100mL1,2-dichloroethane, reacted at room temperature, after 12 hours of reaction, TLC showed that the reaction was complete. The crude product tetrahydropyran-2-ylynhexyl ether was obtained by filtration and concentration, the product weight was 17.5 g, the yield was 96%, and the GC purity was 95.4%, which was directly carried out to the next step reaction.
Embodiment 2
[0030] Add 5-hexyn-1-ol (11.8g, 0.12mol) and 3,4-dihydro-2H-pyran (8.4g, 0.1mol) to the reaction flask, then add 1g of solid acid catalyst ZSM-5 zeolite , 100mL 1,2-dichloroethane, reacted at room temperature, after 12 hours of reaction, TLC showed that the reaction was complete. After filtration and concentration, the crude product tetrahydropyran-2-ylynhexyl ether was obtained, with a product weight of 17.2 g and a GC purity of 81.2%.
Embodiment 3
[0032] Add tetrahydropyran-2-ylynhexyl ether (18.2 g, 0.1 mol) obtained according to the method of Example 1 into the reaction flask, vacuumize, fill with nitrogen three times, then add 50 mL of dry tetrahydrofuran, stir to dissolve, and cool to -20°C, then a solution of n-butyllithium in pentane (2.5M, 80 mL, 0.2 mol) was added dropwise, and stirring was continued for half an hour. Add hexanal (11g, 0.11mol) tetrahydrofuran (50mL) dropwise to the reaction solution. After the dropwise addition, react at -20°C for two hours, then stir at room temperature for 12 hours. TLC shows that the reaction is complete. The aqueous solution of saturated ammonium chloride was added dropwise to quench, then ethyl acetate was added for extraction, washed twice with saline, and the organic phase was dried with anhydrous magnesium sulfate, filtered, solvent removed, and rectified under reduced pressure. The condensation intermediate was obtained, the product weight was 25.9 g, the yield was 91....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com