A class of 4-pyrimidinediamine small molecule organic compounds and derivatives thereof, and applications thereof
An organic compound, ‐pyrimidinediamine technology, applied in the field of 4‐pyrimidinediamine small molecule organic compounds, can solve the problems of limited research and development, high cost, poor affinity, etc., and achieve the goal of inhibiting proliferation, promoting apoptosis, and improving activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Embodiment 1: Preparation of 4-pyrimidinediamine small molecule organic compound
[0137] Example 1-01, N 2 ‐Ethylamino‐N 4 Preparation of -(3‐chloro‐4‐methoxyphenyl)‐5‐fluoro‐2,4‐pyrimidinediamine (WH‐001)
[0138] Take 3‐chloro‐4‐methoxyaniline (1.567g, 10.0mmol) in absolute ethanol (30mL), add 5‐fluoro‐2,4‐dichloropyrimidine (1.837g, 11.0mmol) and 5.2mL of DIEA, heated to 40°C and stirred overnight. Post-processing: remove most of the organic solvent under reduced pressure, extract three times with dichloromethane and water, combine the organic phases and dry with anhydrous sodium sulfate, remove dichloromethane under reduced pressure, and obtain intermediate 2-chloro ‐5‐fluoro‐N‐(3‐chloro‐4‐methoxyphenyl)pyrimidin‐4‐amine (2.707 g, 94%).
[0139] Put 2‐chloro‐5‐fluoro‐N‐(3‐chloro‐4‐methoxyphenyl)pyrimidine‐4‐amine (144.06 mg, 0.5 mmol) in 5 mL of n-butanol, add 2 mL of ethylamine and 0.26mL of DIEA was heated to 120°C and stirred overnight. Post-processing: re...
Embodiment 1-07
[0145] Example 1-07, N 2 ‐((3S,5R)‐3,5‐dimethylpiperazinyl)‐N 4 Preparation of ‐(4‐(6‐bromo‐2‐pyridylamido)phenyl)‐5‐fluoro‐2,4‐pyrimidinediamine (WH‐007)
[0146] Take 4‐nitroaniline (276.26mg, 2.0mmol) in anhydrous DMF (2mL), add 5‐fluoro‐2,4‐dichloropyrimidine (400.7mg, 2.4mmol) and cesium carbonate (2.0g, 6.0mmol ) were reacted overnight at room temperature. Post-processing: extract three times with dichloromethane and water, combine the organic phases and add anhydrous sodium sulfate to dry, remove dichloromethane under reduced pressure, and obtain the intermediate 2‐chloro‐5‐fluoro‐N‐(4‐ Nitrophenyl)pyrimidin‐4‐amine (227 mg, 42.3%).
[0147] Dissolve 2‐chloro‐5‐fluoro‐N‐(4‐nitrophenyl)pyrimidine‐4‐amine (227 mg, 0.85 mmol) in 5 mL of n-butanol, add cis‐2,6‐dimethylpiperene Hazine (289mg, 2.54mmol) and DIEA (0.44mL, 2.54mmol) were heated to reflux at 120°C overnight. Post-processing: most of the solvent was removed under reduced pressure, and after routine processin...
Embodiment 2
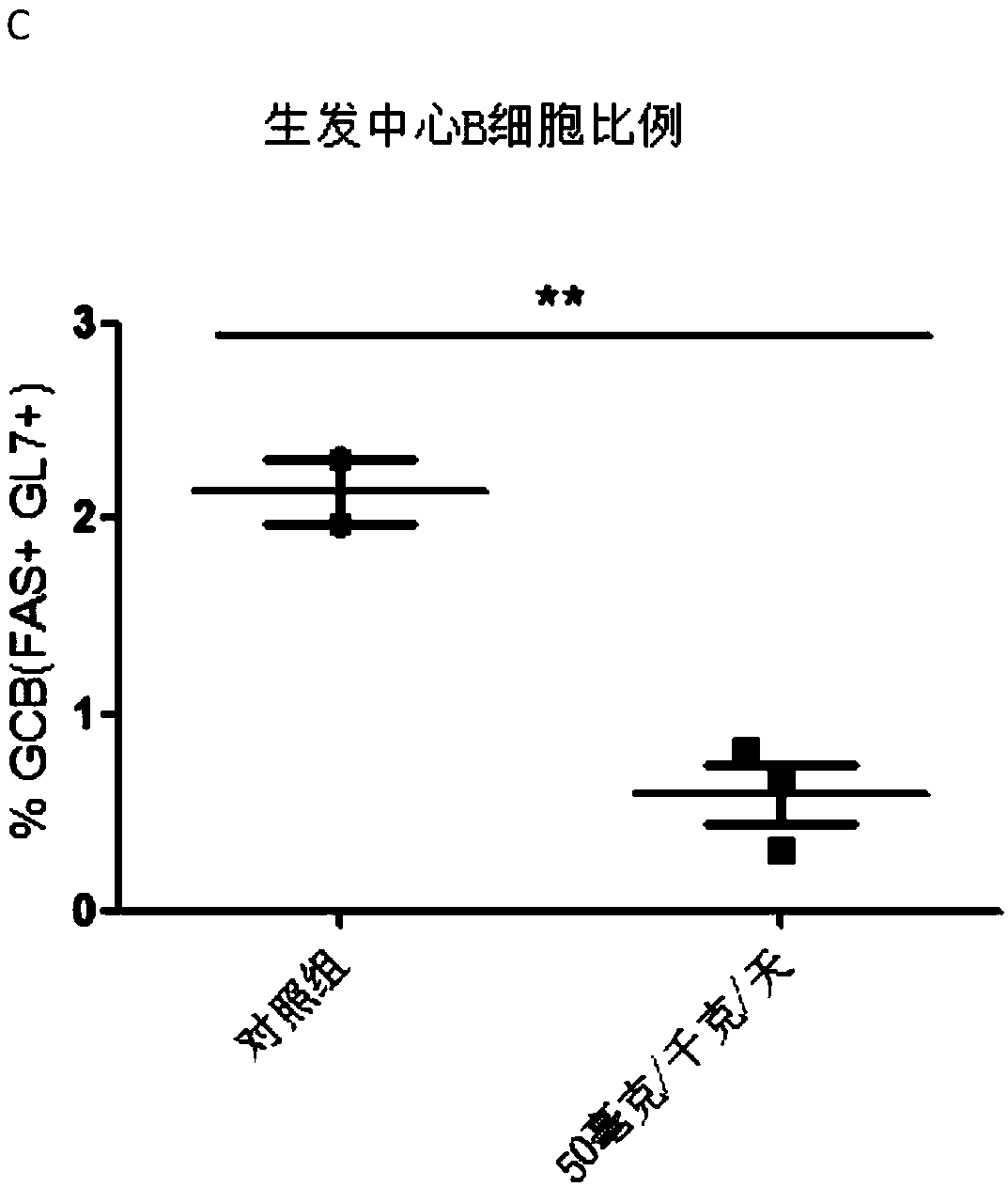
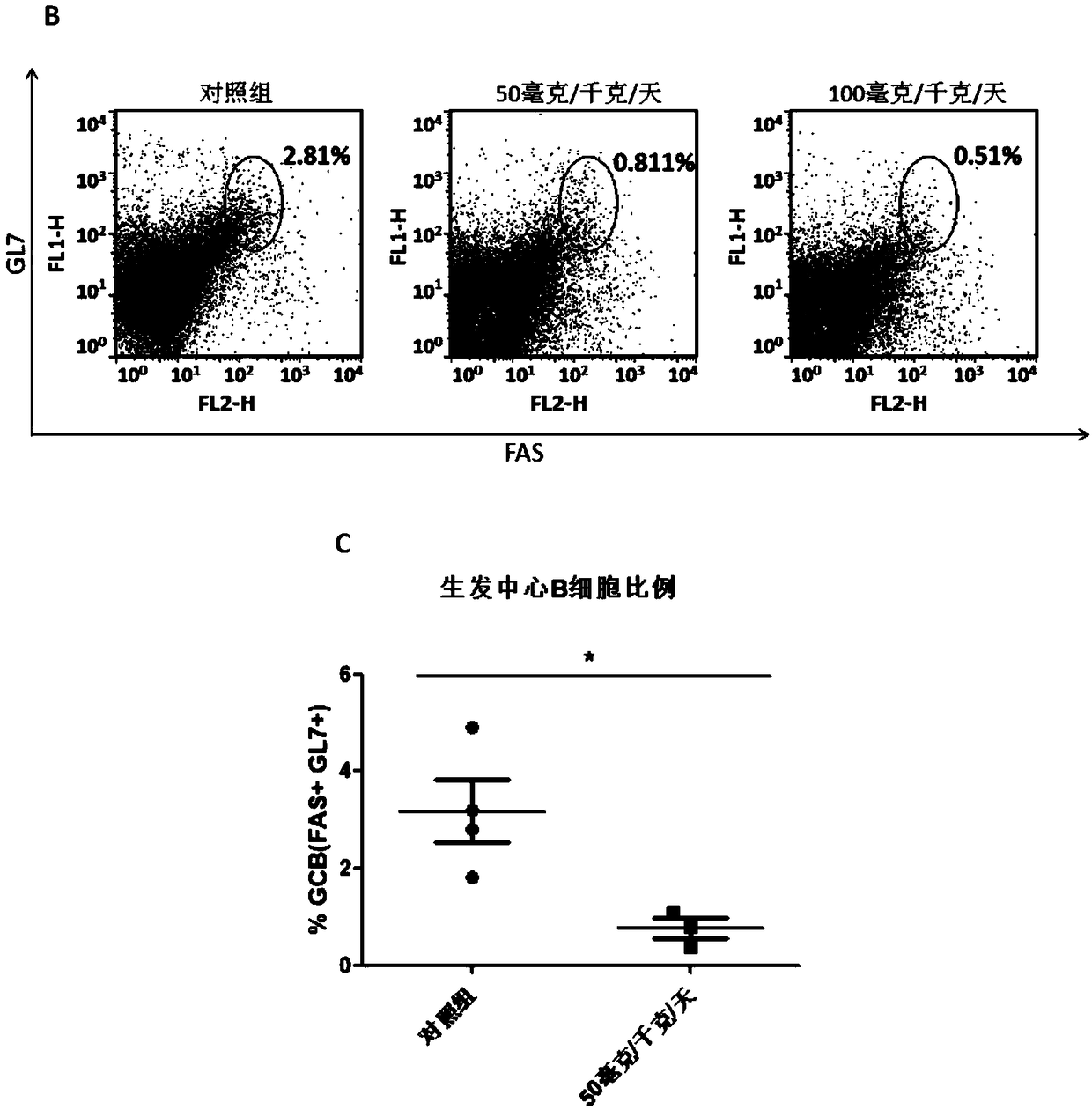
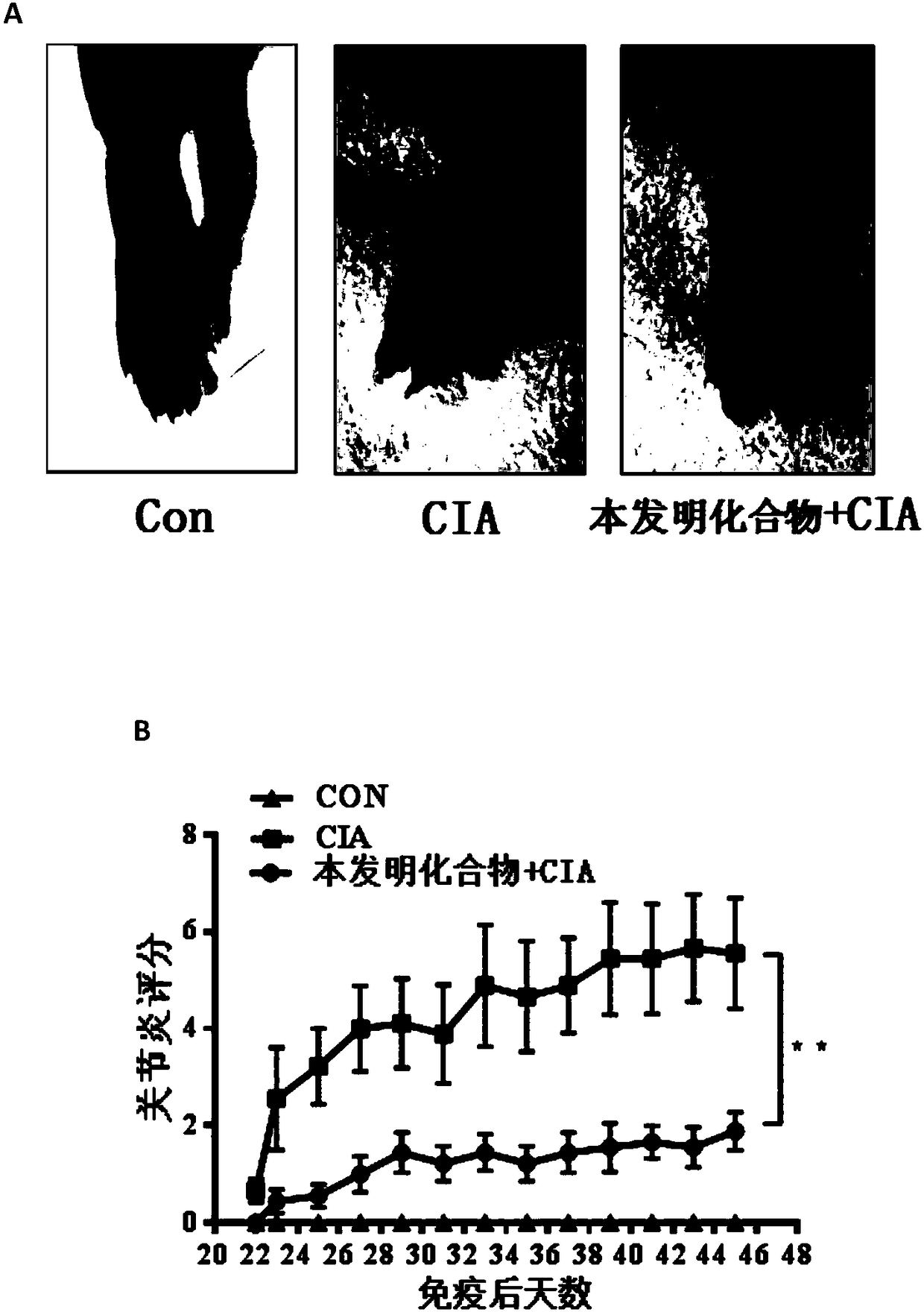
[0169] Example 2: Homogeneous time-resolved fluorescence technique (HTRF) detects the compound of the present invention inhibits the interaction between BCL6 fragment protein and its co-repressor factor SMRT
[0170] Technical method:
[0171] Homogeneous time-resolved fluorescence (HTRF) is a method for detecting analytes in pure liquid phase systems that combines two techniques, fluorescence resonance energy transfer (FRET) and time-resolved fluorescence (TRF). In this system, the selected energy donor of the present invention is GST-Tb, and the acceptor is 6His-XL665 (Cisbio). Correspondingly, the expressed and purified proteins of the present invention also bear GST and His tags respectively, namely BCL6-GST and 6His-SMRT. The transcriptional repression active domain BTBdomain of BCL6 is a domain that binds to the transcription co-repressor SMRT (polypeptide SMRT). The BTB / POZ domain sequence at the N-terminal of the BCL6 protein required in the present invention is ADSC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com