A preparing method of deuterated aromatic compounds
An aromatic compound, deuterated technology, applied in the preparation of organic compounds, oxygenated compounds, carbon-based compounds and other directions, can solve the problems of poor functional group compatibility, inconvenient operation, limited application value, etc., and achieve mild conditions, The effect of reducing production costs and suitable for mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
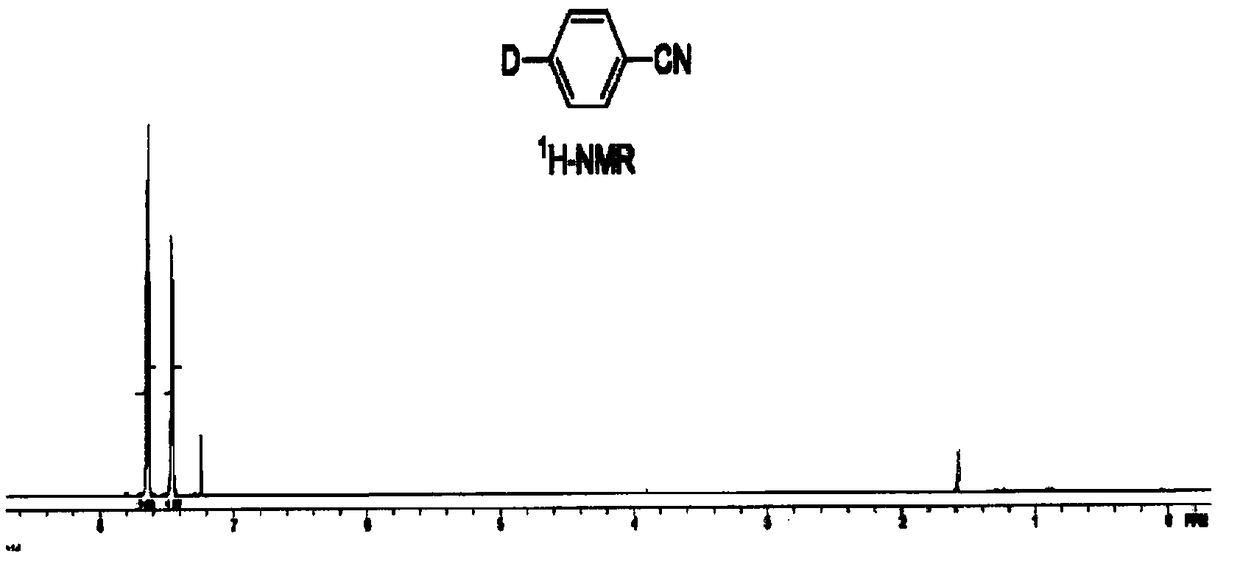
[0029] Embodiment 1: the preparation method of 4-deuterated ethyl benzoate
[0030] Add bis(dibenzylideneacetone)palladium (183mg, 0.2mmol), triphenylphosphine (105mg, 0.4mmol), sodium deuteroformate (1.38g, 20mmol) and ethyl p-bromobenzoate successively into a 100mL three-necked flask Ester (2.28 g, 10 mmol) and dimethylsulfoxide (10 mL). Replaced with high-purity nitrogen three times, reacted at 80oC for 8 hours, added saturated ammonium chloride solution to quench the reaction, extracted the mixed solution with dichloromethane, combined the organic phase, concentrated the organic solvent, and further purified the product by column chromatography to obtain 4-deuterated Ethyl benzoate, yield 95%. Deuteration rate>98%.
[0031] 1H NMR (CDCl3, ppm): δ8.02(d, J=10.2Hz, 2H), 7.41(d, J=10.2Hz, 2H), 4.36(d, J=8.4Hz, 2H), 1.38(t, J = 7.8 Hz, 3H); 13C NMR (CDCl3, ppm): δ 166.6, 132.5 (t, J = 23.75 Hz), 130.5, 129.5, 128.2, 60.9, 14.3.
Embodiment 2
[0032] Embodiment 2: the preparation method of 4-deuterated benzoic acid
[0033] Add bis(dibenzylideneacetone)palladium (183mg, 0.2mmol), triphenylphosphine (105mg, 0.4mmol), sodium deuteroformate (2.07g, 30mmol), p-bromobenzoic acid ( 2.01g, 10mmol) and dimethylsulfoxide (10mL). Replaced with high-purity nitrogen three times, reacted at 80oC for 8 hours, added saturated ammonium chloride solution to quench the reaction, extracted the mixed solution with dichloromethane, combined the organic phase, concentrated the organic solvent, and further purified the product by column chromatography to obtain 4-deuterated Benzoic acid, yield 85%. Deuteration rate>98%. 1H NMR (CDCl3, ppm): δ8.02 (d, J = 10.2Hz, 2H), 7.41 (d, J = 10.2Hz, 2H); 13C NMR (CDCl3, ppm): δ166.6, 132.5 (t, J = 23.75Hz), 130.5, 129.5, 128.2;
Embodiment 3
[0034] Embodiment 3: the preparation method of 4-deuterated benzophenone
[0035] Add bis(dibenzylideneacetone)palladium (183mg, 0.2mmol), triphenylphosphine (105mg, 0.4mmol), sodium deuteroformate (1.38g, 20mmol), 4-bromodiphenyl Methanone (2.60 g, 10 mmol) and dimethylsulfoxide (10 mL). Replaced with high-purity nitrogen three times, reacted at 80oC for 8 hours, added saturated ammonium chloride solution to quench the reaction, extracted the mixed solution with dichloromethane, combined the organic phase, concentrated the organic solvent, and further purified the product by column chromatography to obtain 4-deuterated Benzophenone, yield 90%. Deuteration rate>98%. 1H NMR (CDCl3, ppm): δ7.78~7.80 (m, 4H), 7.57 (t, J=9.0Hz, 1H), 7.45~7.48 (m, 4H); 13C NMR (CDCl3, ppm): δ196. 8, 137.7, 132.5, 132.2 (t, J = 25.0Hz), 130.1, 128.4, 128.2;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com