Triazole compound, synthesis method and application thereof
A compound, triazole technology, applied in the field of biomedicine, can solve problems such as adverse reactions, long-term use of high-dose selective COX2 inhibitors, and side effects are not recommended
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
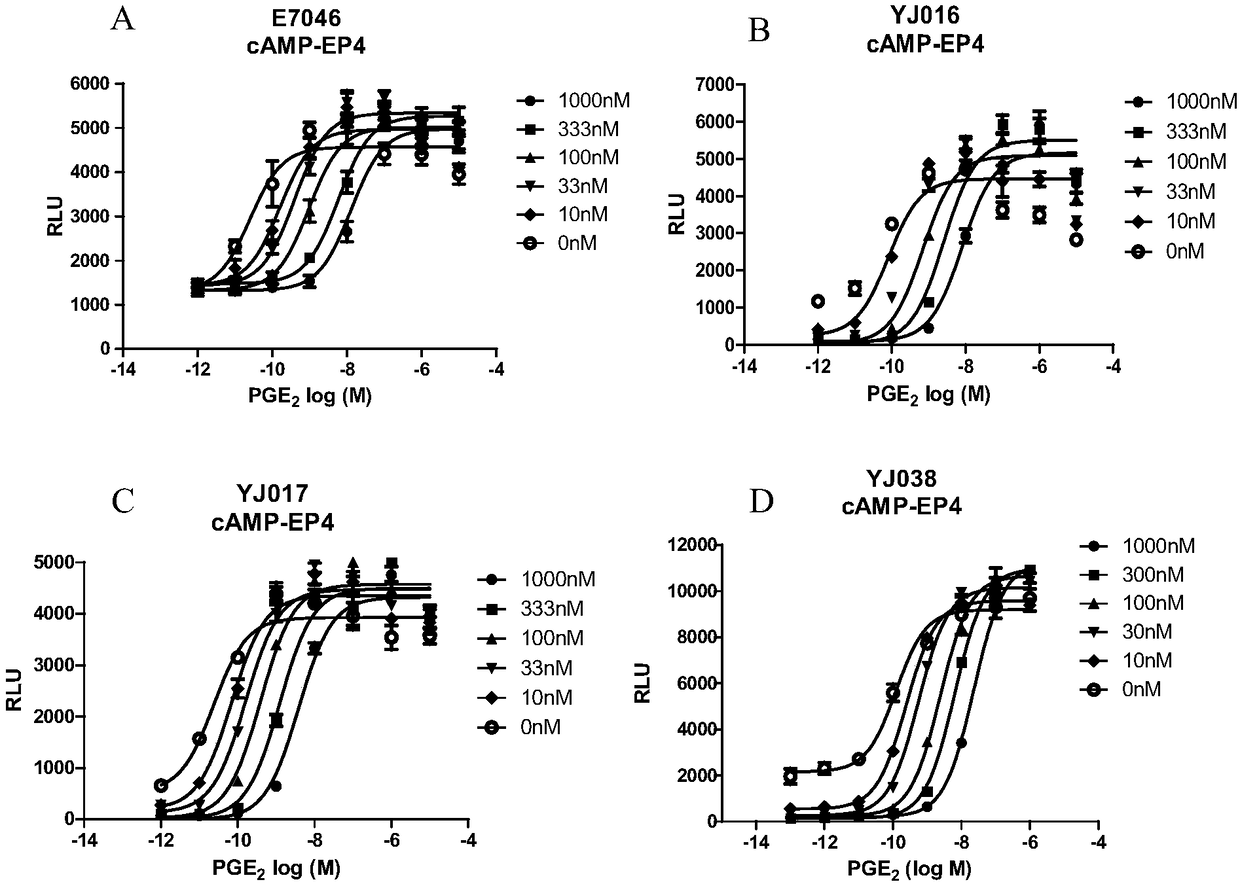
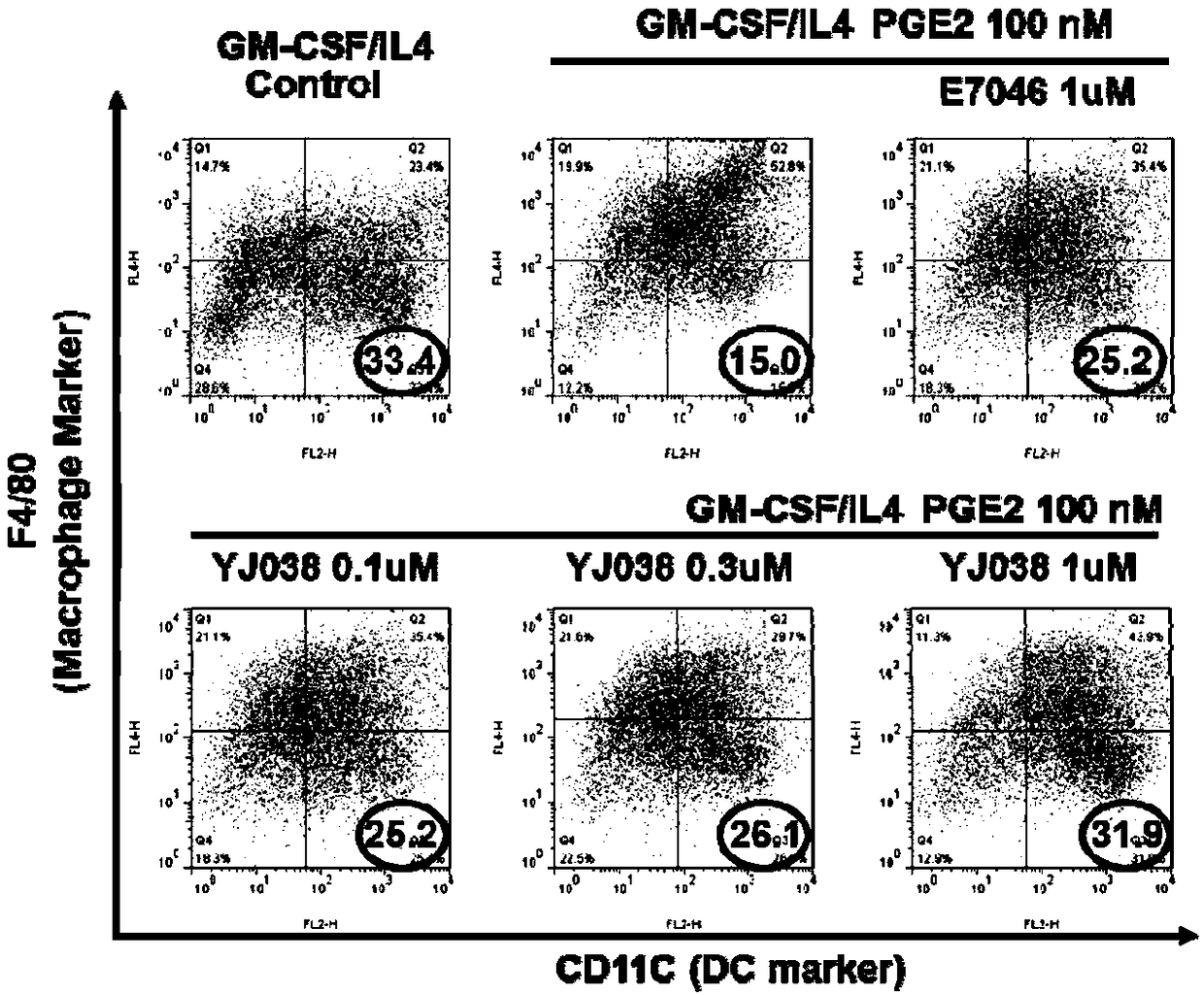
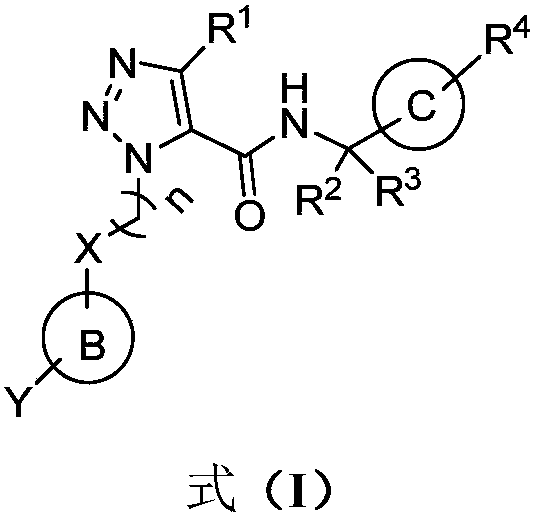
Image
Examples
Embodiment 1-1
[0116] Example 1-1, Compound (S)-4-(1-(1-(4-(trifluoromethyl)benzyl)-4-methyl-1hydrogen-1,2,3-triazole-5 - Preparation of formamido) ethyl) benzoic acid (YJ001)
[0117]
[0118] Take 4-trifluoromethylbenzyl bromide (717mg, 3mmol) in anhydrous dimethyl sulfoxide (4ml), add sodium azide (215mg, 3.3mmol), and stir at room temperature for 24h. After the reaction was completed, it was extracted with ethyl acetate, and the crude product 4-trifluoromethylbenzyl azide (556 mg, 92%) was obtained after conventional treatment.
[0119] Dissolve 4-trifluoromethylbenzyl azide (556mg, 2.77mmol) in anhydrous toluene (5ml), add ethyl 2-butynoate (224mg, 2mmol), react at 120°C for 12h and cool to room temperature. Most of the solvent was removed under reduced pressure, and after conventional treatment, it was passed through a silica gel column to obtain the intermediate 1-(4-(trifluoromethyl)benzyl)-1H-1,2,3-triazole-4-methyl-5- Ethyl formate (210 mg, 35%).
[0120] Dissolve ethyl 1-(4...
Embodiment 1-37
[0128] Example 1-37, Compound (S)-4-(1-(1-(4-(trifluoromethyl)benzyl)-4-(4-fluorophenyl)-1hydrogen-1,2,3 - Preparation of triazole-5-carboxamido) ethyl) benzoic acid (YJ037)
[0129]
[0130]Take 4-trifluoromethylbenzyl bromide (23.9g, 100mmol) in anhydrous dimethyl sulfoxide (30ml), add sodium azide (7.15g, 110mmol), and stir at room temperature for 24h. After the reaction was completed, it was extracted with ethyl acetate, and the crude product 4-trifluoromethylbenzyl azide (18.492 g, 92%) was obtained after conventional treatment.
[0131] Dissolve ethyl propiolate (9.8g, 100mmol) in acetone (30ml), add silver nitrate (1.7g, 10mmol), and react with N-bromosuccinimide (19.58g, 110mmol) at room temperature for 12h , most of the solvent was removed under reduced pressure, and after routine treatment, it was passed through a silica gel column to obtain ethyl bromopropiolate (17.6 g, 99%).
[0132] Dissolve 4-trifluoromethylbenzyl azide (18.492g, 92mmol) in anhydrous toluen...
Embodiment 1-45
[0141] Example 1-45, compound (S)-4-(1-(5-(trifluoromethyl)-1-(4-(trifluoromethyl)benzyl)-1H-1,2,3-tri Preparation of azole-4-carboxamido)ethyl)benzoic acid (YJ045)
[0142]
[0143] Take 4-trifluoromethylbenzyl bromide (717mg, 3mmol) in anhydrous dimethyl sulfoxide (4ml), add sodium azide (215mg, 3.3mmol), and stir at room temperature for 24h. After the reaction was completed, it was extracted with ethyl acetate, and the crude product 4-trifluoromethylbenzyl azide (556 mg, 92%) was obtained after conventional treatment.
[0144] Dissolve 4-trifluoromethylbenzyl azide (556mg, 2.77mmol) in anhydrous toluene (5ml), add 4,4,4-trifluoro-2-butyne ethyl ester (332mg, 2mmol), in After reacting at 120°C for 12h, it was cooled to room temperature. Most of the solvent was removed under reduced pressure, and the silica gel column was passed after conventional treatment to obtain the intermediate ethyl 5-(trifluoromethyl)-1-(4-(trifluoromethyl)benzyl)-1H-1,2,3 - Ethyl triazole-4-car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com