Preparation method for fluazinam
A technology based on trifluoromethylpyridine and pyridyl, applied in the field of compound synthesis, can solve the problems of limited range of solvent selection, increase of waste water treatment cost, increase of alkali removal workload, etc., to suppress hydrolysis side reactions and benefit environmental protection , improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
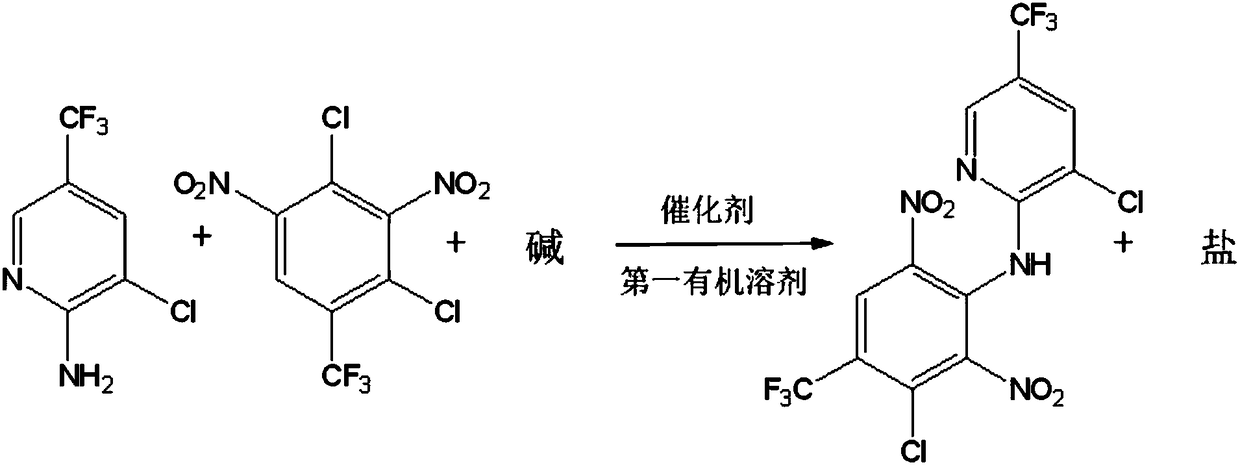
[0034] A 3-chloro-N-(3-chloro-5-trifluoromethyl-2-pyridyl)-α,α,α-trifluoro-2,6-dinitro-para The preparation method of toluidine, comprises the following steps:
[0035] (1) Make 2-amino-3-chloro-5-trifluoromethylpyridine and 2,4-dichloro-3,5-dinitrobenzotrifluoride in the presence of the first organic solvent, alkali and catalyst Specify the reaction temperature to react to obtain a reaction mixture;
[0036] (2) Purified 3-chloro-N-(3-chloro-5-trifluoromethyl-2-pyridyl)-α,α,α-trifluoro- 2,6-Dinitro-p-toluidine.
[0037] In step (1), 2-amino-3-chloro-5-trifluoromethylpyridine is condensed with 2,4-dichloro-3,5-dinitrobenzotrifluoride, and its specific reaction formula is as follows:
[0038]
[0039] In step (1), relative to 1 mol of 2-amino-3-chloro-5-trifluoromethylpyridine, 1.0-1.2 mol of 2,4-dichloro-3,5-dinitrotrifluoro toluene. Preferably, 1.0-1.05 mol of 2,4-dichloro-3,5-dinitrobenzotrifluoride may be used relative to 1 mol of 2-amino-3-chloro-5-trifluoromethylp...
Embodiment 1
[0063] 200 g (i.e., about 1 mol) of 2-amino-3-chloro-5-trifluoromethylpyridine with a purity of 99%, 500 ml of acetonitrile, 1.5 g (i.e., about 0.004 mol) of ethylenediamine tetramine with a purity of 99% Disodium acetate Na 2 EDTA and 200g (that is, about 3.03mol) purity are 85% solid potassium hydroxide and drop into in the four-necked flask that is equipped with stirrer, thermometer and dropping funnel, open stirring and mix, be cooled to 0-5 ℃, then Add dropwise a solution containing 314.5g (i.e., about 1.03mol) of 2,4-dichloro-3,5-dinitrobenzotrifluoride and 500ml of acetonitrile with a purity of 99%, at a temperature of 0-5°C; After the addition was complete, the reaction was continued at 0-5°C for 3 hours.
[0064] After the reaction was completed, filter at normal temperature, and rinse the filter cake twice with acetonitrile, using 50ml of acetonitrile each time. After combining the obtained filtrate and eluent, it was neutralized to pH 6 with a small amount of 98% ...
Embodiment 2
[0066] 200g (i.e. about 1 mol) of 99% purity of 2-amino-3-chloro-5-trifluoromethylpyridine, 300ml of acetonitrile, 2.2g (i.e. about 0.097mol) of 99% purity of benzyl triethyl Ammonium chloride TEBA and 146g (that is, about 3.5mol) of solid sodium hydroxide with a purity of 96% are put into a four-necked flask equipped with a stirrer, a thermometer and a dropping funnel, and the stirring is started and mixed, cooled to 5- 10°C, then dropwise add a solution containing 305.3g (that is, about 1.00mol) of 2,4-dichloro-3,5-dinitrobenzotrifluoride and 200ml of acetonitrile with a purity of 99%, at a temperature of 5- 10°C; after the dropwise addition, continue the reaction at 5-10°C for 5 hours.
[0067] After the reaction was completed, filter at normal temperature, and rinse the filter cake twice with acetonitrile, using 50ml of acetonitrile each time. After combining the obtained filtrate and eluent, it was neutralized to pH 4 with a small amount of 98% concentrated sulfuric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com