Recombinant plasmid, DNA vaccine, preparation method and application thereof
A DNA vaccine and recombinant plasmid technology, applied in the field of genetic engineering, can solve the problems of lack of specific treatment methods, achieve good therapeutic effect, reduce the degree of colonic mucosal damage, and inhibit the occurrence of inflammation
Inactive Publication Date: 2018-11-27
刘志强 +1
View PDF4 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0003] The current treatment drugs for IBD include salicylic acid preparations, glucocorticoids and some immunosuppressant drugs, but they lack specific treatment methods. Therefore, finding new treatment methods has become a hot and difficult research topic at home and abroad.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
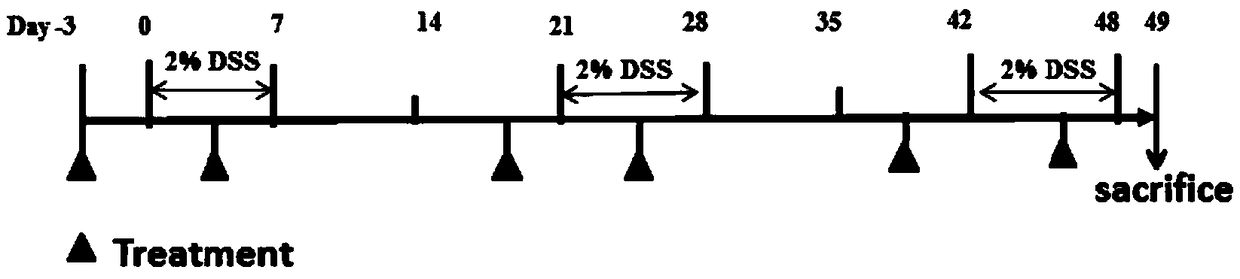
Embodiment 1
[0034] 1. Experimental method
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
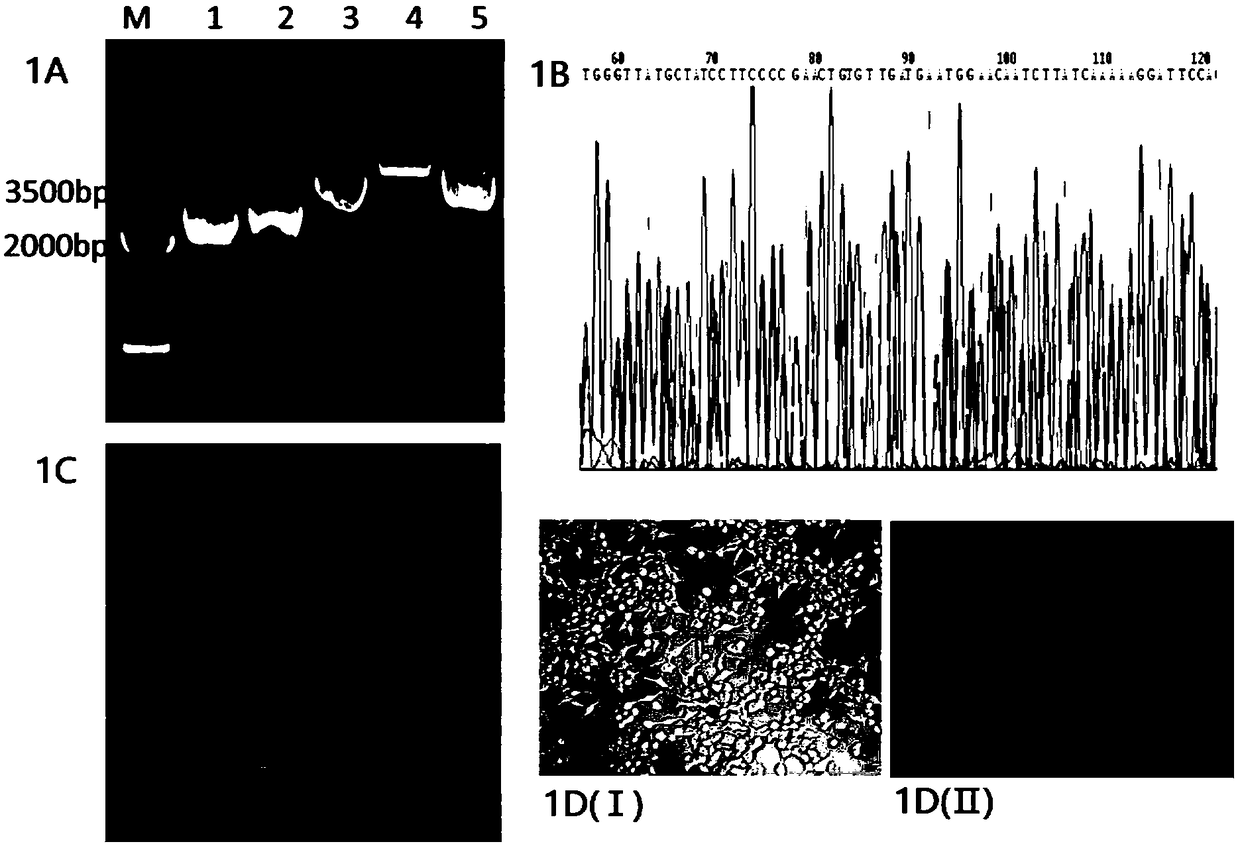
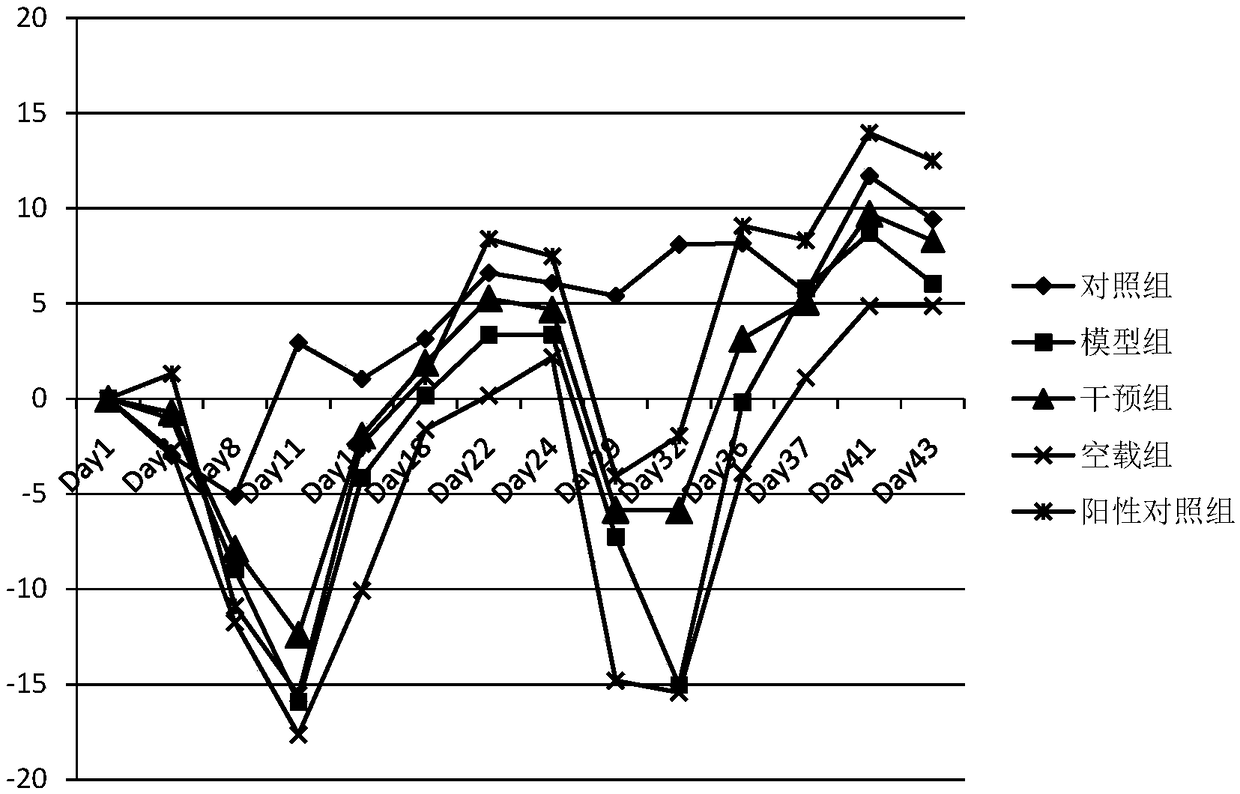
The invention discloses a recombinant plasmid for specifically treating IBD, a DNA vaccine, a preparation method and an application thereof. In an experimental process of the invention, the injury degree of colonic mucosa of an IBD model rat of a treatment group injected with the DNA vaccine is obviously reduced and the score of activity index of enteritidis diseases is lower than that of a modelgroup, but the proinflammatory factors TNF-alpha and IL-6 of intestinal tracts of the model group are increased and T cells of CD4+CD25+Foxp3+ are less than those of pVAX1-TNFAIP3 treatment group. Meanwhile, phosphorylation level of the treatment group associated protein is obviously lower than that of the model group, so that pVAX1-TNFAIP3 is capable of inhibiting inflammation by inhibiting the activation of related inflammation signal channels; besides, the increasing of Foxp3 protein in colon shows that pVAX1-TNFAIP3 is capable of inhibiting inflammation by increasing regulatory T cells; the above results prove that pVAX1-TNFAIP3 recombinant plasmid and the prepared DNA vaccine have excellent therapeutic effects on inflammatory bowel diseases.
Description
technical field [0001] The invention relates to the field of genetic engineering, in particular to a recombinant plasmid, a DNA vaccine and a preparation method and application thereof. Background technique [0002] Inflammatory bowel disease (IBD) is a common chronic non-specific intestinal disease, which can be divided into two types according to clinical and pathological features: ulcerative colitis (UC) and Crohn's disease (Crohn's disease). disease, CD). In recent years, with the improvement of people's living standards and the pollution of the environment, the incidence and prevalence of IBD have shown an obvious increasing trend in my country, which seriously affects the life and work of patients and brings huge burdens to society and economy. The etiology of IBD is not yet clear, but it is generally believed to be related to genetic susceptibility, immune inflammation, intestinal flora, and mental and psychological factors. Among them, the key to the pathogenesis m...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N15/85A61K39/00A61K9/107A61K47/32A61K47/34A61P1/00
CPCA61K9/107A61K39/0005A61K47/32A61K47/34A61K2039/53A61K2039/54A61P1/00C12N15/85C12N2800/107
Inventor 胡田勇胡文会邱书奇耿晓瑞马莉刘江琦刘志强杨平常
Owner 刘志强
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com