Application of composition in preparation of drug for inhibiting gefitinib hepatotoxicity
A technology of gefitinib and composition, applied in the field of application of composition in the preparation of drugs for the treatment of gefitinib hepatotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
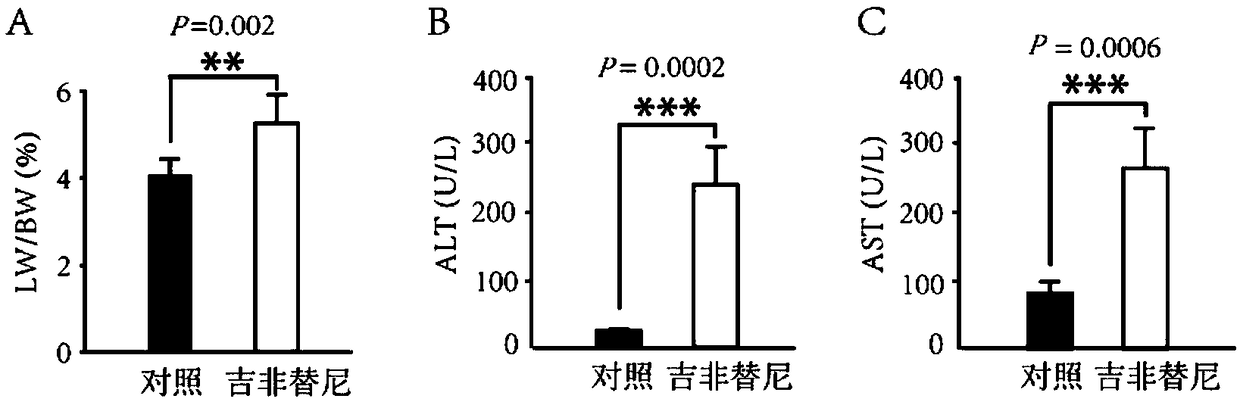
[0013] Twelve female ICR mice were randomly divided into a control group and a gefitinib administration group, each with 6 mice. The control group was given the same amount of 0.9% normal saline; the gefitinib administration group was given gefitinib solution by gavage at a dose of 200 mg / kg / day for 4 consecutive weeks, and the body weight of the mice was recorded every day. After 4 weeks, the mice were sacrificed and their livers were cut out, and their organ coefficients were weighed to calculate their organ coefficients. The eyeballs were removed and blood was taken to detect the ALT and AST values in the serum. The results showed that the liver organ coefficient of the gefitinib administration group was significantly increased, and the ALT and AST values were significantly higher than those of the control group. It shows that gefitinib can cause abnormal liver function in mice and has significant liver toxicity. See results figure 1 .
Embodiment 2
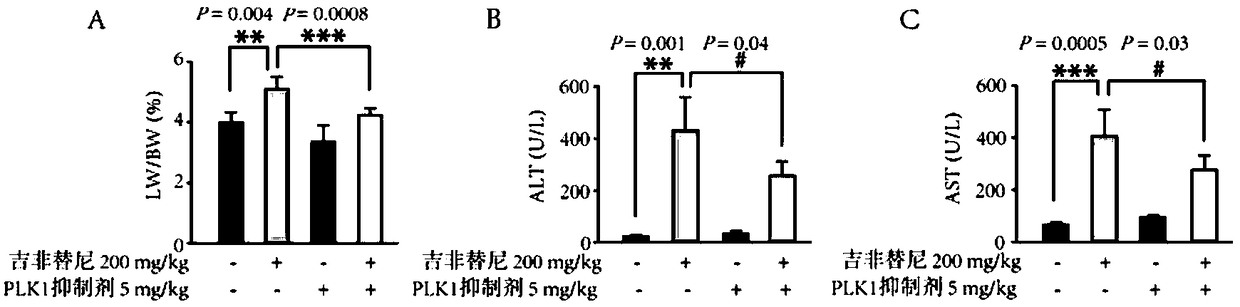
[0015] 24 female ICR mice were randomly divided into 4 groups, namely the blank control group, the gefitinib administration group, the BI-2536 administration group and the gefitinib+BI-2536 administration group, each with 6 mice. Gefitinib solution was administered intragastrically at a dose of 200 mg / kg / day, and / or BI-2536 solution was administered by intraperitoneal injection twice a week at a dose of 5 mg / kg for 4 weeks of continuous administration, and the mice’s performance was recorded daily body weight. After 4 weeks, the mice were sacrificed and their livers were cut out, and their organ coefficients were weighed to calculate their organ coefficients. The eyeballs were removed and blood was taken to detect the ALT and AST values in the serum. It was found that BI-2536 can reverse the increase in liver organ coefficient caused by gefitinib. In addition, the ALT and AST values of the gefitinib single-use group were significantly increased, and BI-2536 can reverse thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com