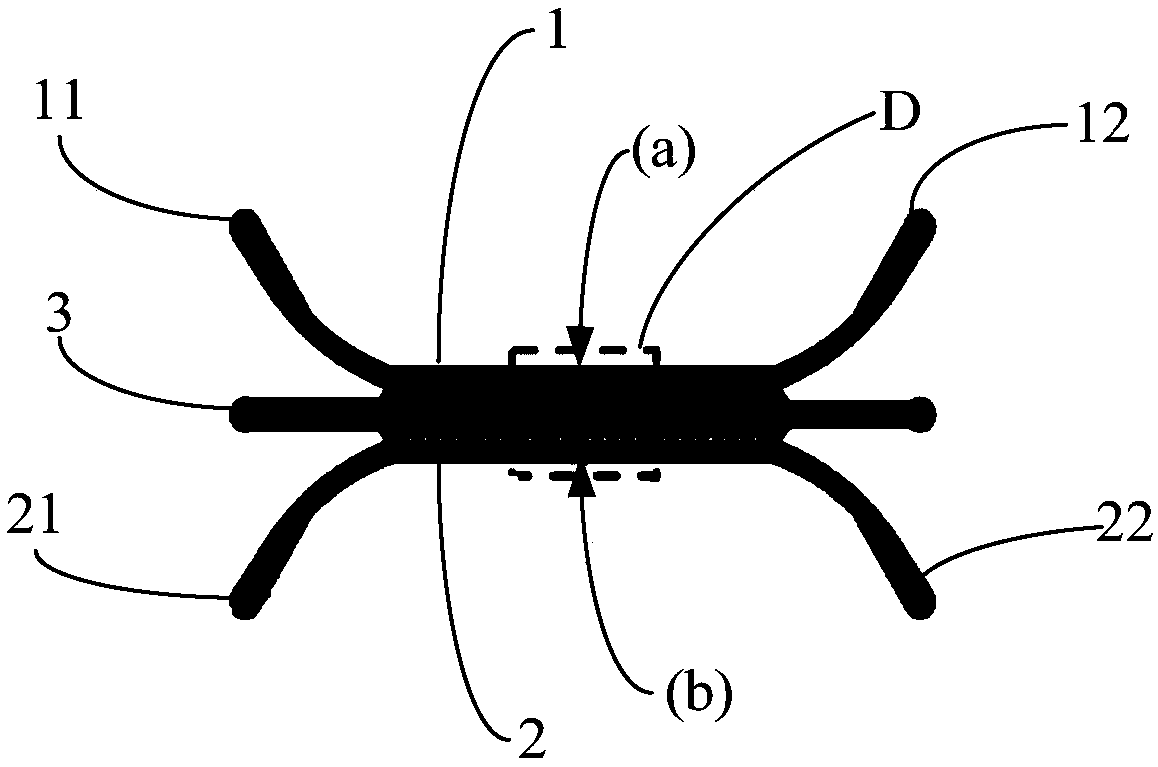
In-vitro construction method for simulating blood brain barrier through human brain microangiogenesis
A blood-brain barrier and construction method technology, applied in artificial cell constructs, biochemical equipment and methods, microorganisms, etc., can solve the problem that 2D models are difficult to obtain in vivo cells, and achieve the goal of reducing sample consumption and reducing test costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Preparation and cultivation of 3D cell culture chip
[0074] (1) Preparation of cell solution: Primary GFP-HBVEC cells (purchased from Science cell, USA) and primary mCherry-HA cells (purchased from Science cell, USA) were respectively used in endothelial cell growth medium EGM-2 (purchased from Lonza) and astrocyte culture medium AM (purchased from Science cell, USA) were resuscitated and cultured. When the cell confluence reached 80-90%, GFP-HBVEC cell suspension and mCherry-HA cell suspension were collected respectively Each 1mL, corresponding to obtain endothelial cell suspension and astrocyte suspension, for use; wherein, the resuscitation culture conditions are: culture temperature 37°C, culture humidity 95%, CO 2 The concentration is 5%; the cell concentration in the GFP-HBVEC cell suspension and mCherry-HA cell suspension are both 1×10 6 cell / mL.
[0075] (2) Preparation of fibrinogen mother liquor: After preheating DPBS (purchased from Gibco, USA) to 37°C,...
Embodiment 2
[0080] Example 2 Morphological characterization of 3D cell culture model
[0081] During the continuous culture period in step (6) of the above-mentioned Example 1, the 3D cell culture chip was scanned by a laser confocal microscope, and 3D fluorescence images were collected for cell morphology identification (GFP green fluorescence was excited at 488nm, and 509nm Emission light imaging; mCherry red fluorescence is excited at 587nm and imaging with 610nm emission light), the 3D fluorescence image acquisition results are as follows Figure 4 to Figure 8 As shown, Figure 4 to Figure 6 These are the 3D fluorescence images collected when the cells are grown on the microfluidic chip for 1, 2, and 3 days. Figure 7 to Figure 8 Is the 3D fluorescence image collected when the cells are grown in the microfluidic chip for 4 days, Figure 7 This is the result of cell growth in the microfluidic chip for 4 days showing the formation of vascular tubule-like structures, Figure 8 This is the re...
Embodiment 3
[0086] Example 3 Functional fluidity verification of 3D cell culture model
[0087] After step (6) of the above-mentioned embodiment 1, particles are loaded into the 3D cell culture chip formed with the brain microvascular network structure, and the trajectory of the loaded particles in the microvascular network is observed by tomography, and the particles are observed The microvascular network moves and migrates through the blood vessels as the fluid flows. The monitoring results of part of its trajectory are as follows Picture 9 Shown ( Picture 9 The granular material shown at C in the middle is partially loaded with particles and evenly distributed in the formed vascular network), indicating that the brain microvascular network formed by endothelial cells and astrocytes exhibits good structural integrity and Vascular permeability.
[0088] In summary, the present invention uses the mixed cells of primary human brain microvascular endothelial cells and primary human brain astro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com