Method for chemically synthesizing diaryl sulfone with asymmetric structure
An asymmetric structure and chemical synthesis technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of less environmental pollution, easy availability of raw materials, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
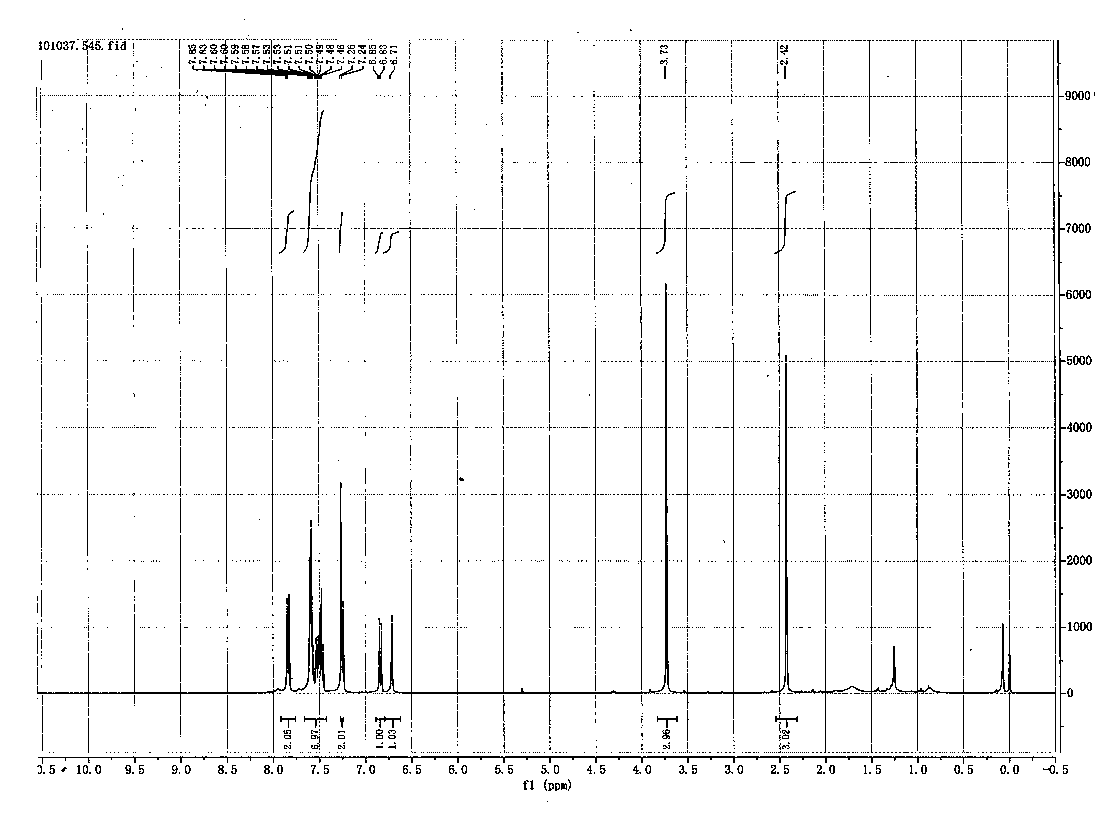
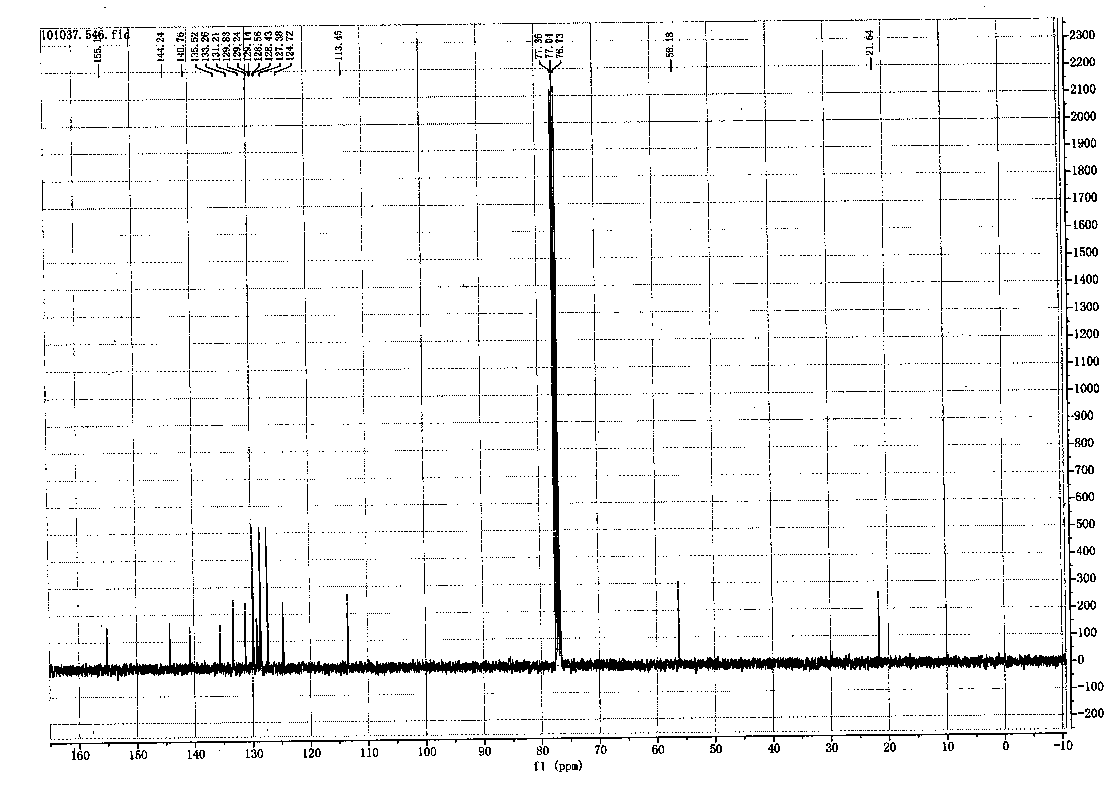
Image
Examples
example 1
[0029] Example 1. How to set the weight range of raw materials:
[0030] (1) Synthesis of quinoneimine monoketal compounds 3 and 5
[0031]
[0032] Aniline compound 1 (R 1 = H,2-Me,2-OMe,3-F,3-OMe;R 2 = Me,Et, i Pr) as the initial raw material (0.020mol, 2.460 g-4.980 g), after dissolving it in pyridine (20mL), adding p-toluenesulfonyl chloride (TsCl) (0.022 mol, 4.180 g) under ice-cooling conditions, adding After completion, move to room temperature to react for 8-12 hours, and stop the reaction after the reaction of raw material 1 is complete. The pyridine was drained, and the crude product was successively washed with 10% hydrochloric acid, saturated NaHCO 3 The solution was extracted with ethyl acetate 3 times (30mL×3), the organic phase was extracted 3 times with saturated brine (30mL×3), and the organic phase was dried with anhydrous sodium sulfate. The solvent was drained, and the crude product was separated by column chromatography (eluent, petroleum ether: et...
example 2
[0038] Example 2. How to set the raw material weight as a certain value:
[0039] The specific implementation steps are as follows (where 3a and 3b are representative compounds of compound 3, and 5a is a representative compound of compound 5):
[0040] (1) Synthesis of quinoneimine monoketals 3a, 3b and 5a
[0041] (a) Substituted aniline compound 1 (R 1 =H;R 2 =Me) as the initial raw material (0.020 mol, 2.460 g), dissolved it in pyridine (20 mL), added p-toluenesulfonyl chloride (TsCl) (0.022 mol, 4.180 g) under ice-cooling conditions, and moved to React at room temperature for 8 hours, and treat raw material 1 (R 1 =H;R 2 =Me) The reaction was completely stopped. The pyridine was drained, and the crude product was successively washed with 10% hydrochloric acid, saturated NaHCO 3 The solution was extracted with ethyl acetate 3 times (30mL×3), the organic phase was extracted 3 times with saturated brine (30mL×3), the organic phase was separated and dried with anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com