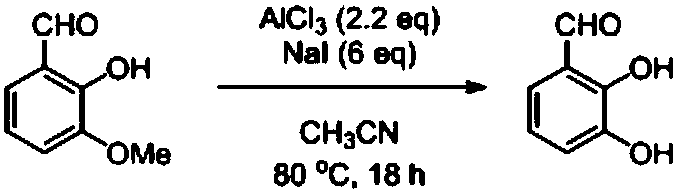Method for preparing 2,3-dihydroxybenzaldehyde by removing methyl of o-vanillin
A technology of dihydroxybenzaldehyde and o-vanillin, which is applied to the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of low yield, high preparation cost, and difficulty in industrialized production , to achieve the effect of simple reaction purification operation, wide source and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Acetonitrile (40ml), aluminum chloride (0.804g, 6.03mmol, 1.1eq), NaI (2.464g, 16.44mmol, 3.0eq) and o-vanillin (0.417g, 2.74mmol) were added to a 100ml eggplant-shaped flask ), heated to 80°C, stopped stirring after 18 hours of reaction, added 2mol / L dilute hydrochloric acid (10ml) to the eggplant-shaped bottle after cooling to room temperature to acidify, extracted with ethyl acetate (50ml×3), combined the organic phases, First wash with a saturated aqueous solution of sodium thiosulfate (10ml), then wash with saturated brine (10ml), dry over anhydrous magnesium sulfate, filter, and the filtrate is evaporated to dryness with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent Purified by ethyl acetate / petroleum ether=1:4, volume ratio) to obtain 0.322g of 2,3-dimethoxybenzaldehyde (yellow solid, yield 85%).
[0022] R f =0.58 (petroleum ether / ethyl acetate=1:3), mp106-108°C.
[0023] 1 H NMR(400MHz,DMSO-d6)δ10.22(s,1...
Embodiment 2
[0025] Add acetonitrile (40ml), aluminum chloride (0.752g, 5.64mmol, 1.1eq), NaI (2.305g, 15.38mmol, 3.0eq) and o-vanillin (0.780g, 5.13mmol) to a 100ml eggplant-shaped flask ), heated to 80°C, stopped stirring after 18 hours of reaction, added 2mol / L dilute hydrochloric acid (10ml) to the eggplant-shaped bottle after cooling to room temperature to acidify, extracted with ethyl acetate (50ml×3), combined the organic phases, First wash with a saturated aqueous solution of sodium thiosulfate (10ml), then wash with saturated brine (10ml), dry over anhydrous magnesium sulfate, filter, and the filtrate is evaporated to dryness with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent Ethyl acetate / petroleum ether=1:4, volume ratio) was purified to obtain 0.620 g of 2,3-dimethoxybenzaldehyde (yellow solid, yield 87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com