Pharmaceutical composition containing esomeprazole sodium and preparation method thereof
A technology of esomeprazole sodium and its composition, which is applied in the field of freeze-dried powder injection containing esomeprazole sodium and its preparation, and can solve the problem of color change of liquid medicine, inability to control impurities, increase of colored impurities, etc. problem, to achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131]
[0132] Prepare freeze-dried powder injection according to the following steps:
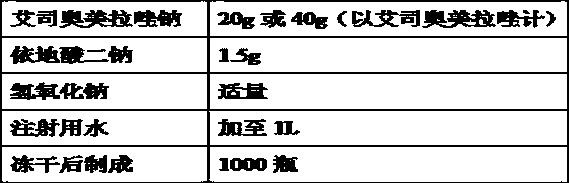
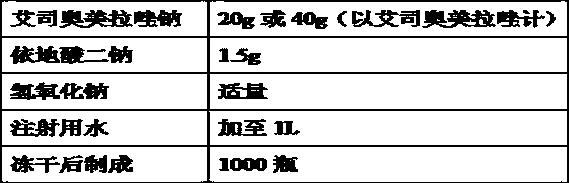
[0133] (1) Prepare 80% of the prescription amount of water for injection at room temperature (10-30°C) in the liquid preparation tank, add the prescribed amount of edetate disodium, stir and dissolve completely at room temperature (10-30°C), use 1mol / L of sodium hydroxide solution, the pH of the liquid is adjusted to 10.7;
[0134] (2) Add the prescribed amount of esomeprazole sodium, stir until it is completely dissolved, add water for injection at room temperature (10-30°C) to make up the volume until the full volume is prepared, stir for 10 minutes after constant volume, and use 1mol / L of hydroxide Sodium solution adjusts the pH of the medicinal solution to 11.5;
[0135] (3) Filter the solution obtained in step (2) through a 0.22 μm sterile filter, fill the filtrate into a neutral borosilicate glass control injection bottle, stopper halfway, and send it to a lyophilizer for lyoph...
Embodiment 2
[0144]
[0145] Prepare freeze-dried powder injection according to the following steps:
[0146] (1) Prepare 60% of the prescription amount of water for injection at room temperature (10-30°C) in the liquid preparation tank, add the prescribed amount of edetate disodium, stir and dissolve completely at room temperature (10-30°C), use 0.5mol / L of sodium hydroxide solution to adjust the pH of the liquid to 11.2;
[0147] (2) Add the prescribed amount of esomeprazole sodium, stir until it is completely dissolved, add water for injection at room temperature (10-30°C) to make up the volume until the full volume is prepared, stir for 10 minutes after constant volume, and use 0.5mol / L hydrogen The sodium oxide solution adjusts the pH of the medicinal solution to 11.0;
[0148] (3) Filter the solution obtained in step (2) through a 0.22 μm sterile filter, fill the filtrate into a neutral borosilicate glass control injection bottle, stopper halfway, and send it to a lyophilizer fo...
Embodiment 3
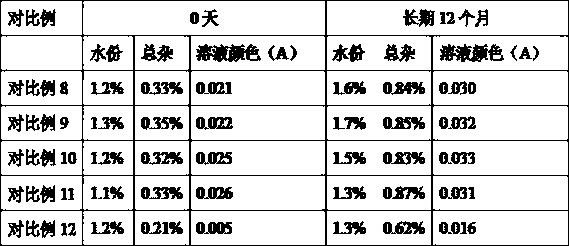
[0157] Samples are prepared according to the prescription described in Example 1, and the liquid preparation method is as follows:
[0158] (1) Prepare 90% of the prescription amount of water for injection at room temperature (10~30°C) in the liquid preparation tank, add the prescribed amount of edetate disodium, stir and dissolve completely at room temperature (10~30°C), use 1.2mol / L of sodium hydroxide solution to adjust the pH of the liquid to 10.7;
[0159] (2) Add the prescribed amount of esomeprazole sodium, stir until completely dissolved, add water for injection at room temperature (10~30°C) to make up the volume until the full volume is prepared, stir for 10 minutes after constant volume, and use 1.2mol / L hydrogen The sodium oxide solution adjusts the pH of the medicinal solution to 11.5;
[0160] (3) Filter the solution obtained in step (2) through a 0.22 μm sterile filter, fill the filtrate into a neutral borosilicate glass control injection bottle, stopper halfwa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com