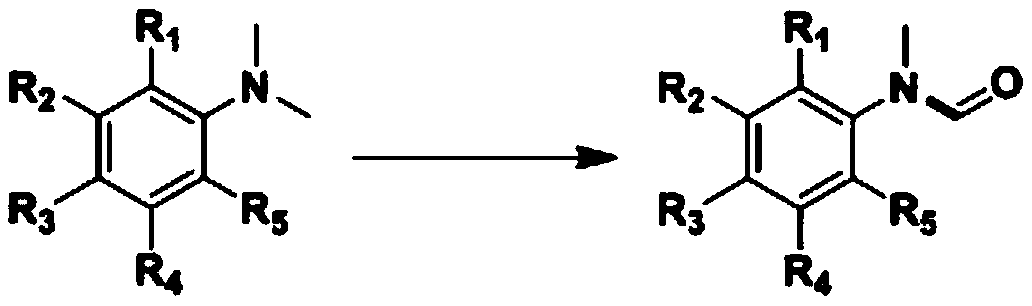
Method for synthesizing N-aryl formamide compound
A technology for aryl formamides and compounds, which is applied in the field of synthesizing N-aryl formamides and their derivatives, can solve the problems of no advantage in atom economy and contrary to environmental protection, achieve good functional group tolerance, reduce environmental pollution, and be good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for synthesizing 4-bromo-N-methylbenzamide, the steps are:
[0028] Step 1: Weighing
[0029] Weigh CuCl1.0mg (0.01mmol), salicylic acid 1.4mg (0.01mmol), NaBF 4 16.5mg (0.15mmol), 4-bromo-N,N-dimethylaniline 200mg (0.01mmol);
[0030] Step 2: Weigh 1.0 mL of acetonitrile and add it to the Scheck tube, and add the CuCl, salicylic acid, NaBF 4 and 4-bromo-N,N-dimethylaniline were put into acetonitrile, and stirred at 30°C for 48 hours under an oxygen atmosphere. After the reaction was completed, 10 mL of distilled water was added to the mixture, and then extracted three times with 15 mL of dichloromethane, and collected Organic phase, with anhydrous Na 2 SO 4 Dry, filter with suction, remove dichloromethane with a rotary evaporator, and separate by column chromatography to obtain a white solid 4-bromo-N-methylbenzamide with a yield of 60%, a white solid, m.p.61.1-66.1°C. Spectral data is: Main structure: 1 H NMR (400MHz, CDCl 3 )δ: 8.45(s, 1H), 7.52(d, J=...
Embodiment 2
[0032] A kind of method of synthesizing 4-chloro-N-methylbenzamide, its step is
[0033] Step 1: Weighing
[0034] Weigh CuCl1.0mg (0.01mmol), salicylic acid 1.4mg (0.01mmol), NaBF 4 16.5mg (0.15mmol), 4-chloro-N,N-dimethylaniline 200mg (0.01mmol);
[0035] Step 2: Weigh 1.0 mL of acetonitrile and add it to the Scheck tube, and add the CuCl, salicylic acid, NaBF 4 and 4-chloro-N,N-dimethylaniline were put into acetonitrile, and stirred at 30°C for 45 hours under an oxygen atmosphere. After the reaction was completed, 10 mL of distilled water was added to the mixture, and then extracted three times with 15 mL of dichloromethane, and collected Organic phase, with anhydrous Na 2 SO 4 Drying, suction filtration, removal of dichloromethane with a rotary evaporator, separation by column chromatography to obtain a white solid 4-chloro-N-methylbenzamide, the yield is 63%, and the spectral data are: 1 H NMR (400MHz, CDCl 3 )δ: 8.46(s, 1H), 7.53(d, J=8.0Hz, 2H), 7.06(d, J=8.8Hz, 2...
Embodiment 3
[0038] A kind of method for synthesizing 4-fluoro-N-methylbenzamide, its step is
[0039] Step 1: Weighing
[0040] Weigh CuCl1.0mg (0.01mmol), salicylic acid 1.4mg (0.01mmol), NaBF 4 16.5mg (0.15mmol), 4-fluoro-N,N-dimethylaniline 200mg (0.01mmol);
[0041] Step 2: Weigh 1.0 mL of acetonitrile and add it to the Scheck tube, and add the CuCl, salicylic acid, NaBF 4 and 4-fluoro-N,N-dimethylaniline were put into acetonitrile, stirred at 35°C for 40 hours under an oxygen atmosphere, and the reaction was completed; 10 mL of distilled water was added to the mixture, and extracted three times with 15 mL of dichloromethane, Organic phase, with anhydrous Na 2 SO 4 Dry, filter with suction, remove methylene chloride with a rotary evaporator, and separate by column chromatography to obtain oily 4-fluoro-N-methylbenzamide, with a yield of 52%, and the spectral data are: 1 H NMR (400MHz, CDCl 3 )δ: 8.37(s, 1H), 7.15-7.05(m, 4H), 3.28(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ: 162.2, 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com