Application of IgD-Fc-Ig fusion protein in preparation of drugs for treating acute lymphocytic leukemia
An acute lymphocyte, igd-fc-ig technology, applied in the field of IgD-Fc-Ig fusion protein, can solve the problems of unclear IgD/IgDR-related functions and few researches on IgD/IgDR-related signaling pathways, and achieve stability. Good sex, improve half-life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
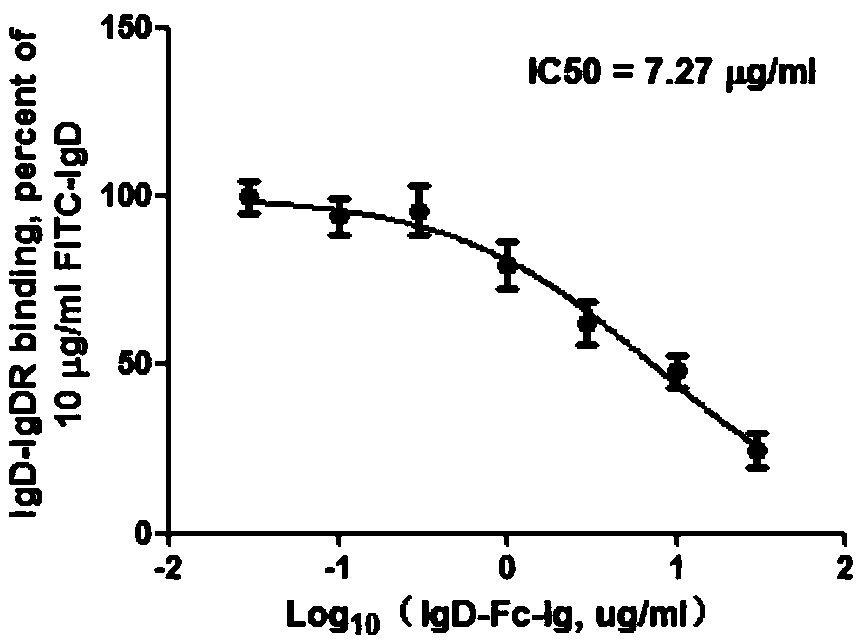
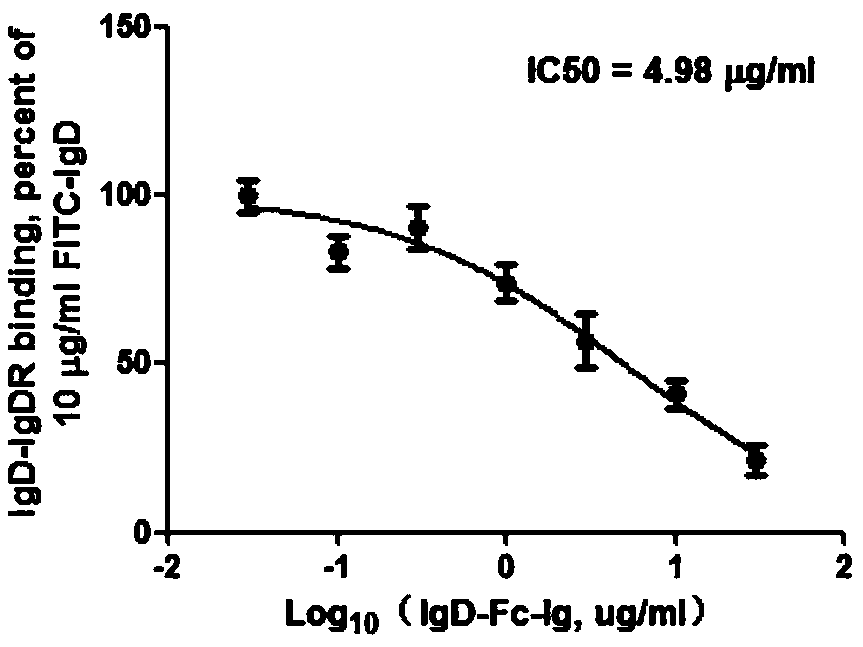
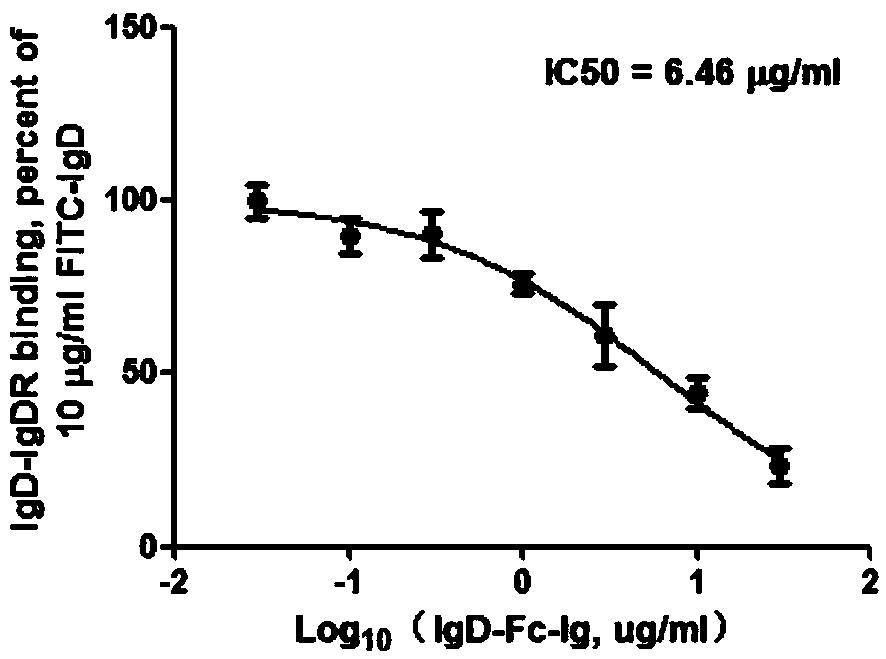
[0023] Embodiment 1: IgD, IgD-Fc-Ig fusion protein competition binding experiment
[0024] 1.1 Experimental method
[0025] CD4+ T cells, Jurkat and MOLT-4 cells were all treated with 2×10 6 The cell density per well was spread in 6-well plate, and FITC-IgD (10 μg / ml) and different concentrations of IgD-Fc-Ig fusion protein (0.03, 0.1, 0.3, 1, 3, 10, 30 μg / ml) were added respectively, 37 Incubate at ℃ for 2h. Wash twice with PBS, 300g×10min, discard the supernatant, resuspend the cells with an appropriate amount of PBS, and detect the fluorescence intensity on the machine. The binding properties of ligand IgD, IgD-Fc-Ig fusion protein and IgDR were calculated by Fluorescent Intensity (FI) values. According to the results of flow cytometry, the competition binding curve was drawn with different concentrations of IgD-Fc-Ig fusion protein as the X axis and the corresponding IgD specific binding amount as the Y axis. According to the binding curve, the IC of IgD-Fc-Ig fusion p...
Embodiment 2
[0028] Embodiment 2: CCK-8 method detects IgD-Fc-Ig fusion protein to the CD4 stimulated by IgD + Effects on Cell Viability of T Cells and T-ALL Cell Lines
[0029] 2.1 Experimental method
[0030] 2.1.1 Detection of CD4 by CCK-8 method + T cell activity
[0031] 20ml of peripheral blood was extracted from healthy volunteers, treated with EDTA-K2 for anticoagulation, PBMC was separated by Ficoll density method, and CD4 was sorted by magnetic beads + T cells, and adjust the cell density to 2×10 6 / ml, add 100 μl to each well and inoculate in a 96-well culture plate. Set: blank control group (Control group), IgD group (final concentration is 3 μg / ml), IgD-Fc-Ig fusion protein single use group (0.3, 1, 3, 10, 30 μg / ml), IgD-Fc-Ig Fusion protein, IgD combined group (0.3, 1, 3, 10, 30 μg / ml+3 μg / ml IgD) and IgG-Fc recombinant protein (10 μg / ml) were placed at 37°C, 5% CO 2 Cultivate in the incubator for 24h. After terminating the culture, add 10 μl of CCK-8 reagent to each w...
Embodiment 3
[0037] Example 3: Flow cytometry detection of IgD-Fc-Ig fusion protein to IgD-stimulated CD4 + Influence of Apoptosis on T Cells and T-ALL Cell Lines
[0038] 3.1 Experimental method
[0039] CD4 + cells or Jurkat cells in 10 6 Inoculated in a 6-well plate per ml, stimulated with IgD (3 μg / ml), and added different concentrations of IgD-Fc-Ig fusion protein at the same time, at 37 ° C, 5% CO 2 After culturing in a cell culture incubator for 24 hours, collect the cells by centrifugation, wash twice with PBS, resuspend the cells in 500 μl 1×Binding Buffer, add 5 μl Annexin V-FITC and 10 μl PI to each tube, vortex gently, and incubate for 5 minutes at room temperature in the dark . On-board detection by flow cytometry.
[0040] 3.2 Experimental results
[0041] 3.2.1 The applicant has tested IgD against CD4 + The influence of T cell apoptosis and the effect of IgD-Fc-Ig, IgD (3 μg / ml) stimulation group, IgG (10 μg / ml) negative control group, IgD (3 μg / ml) + IgD-Fc-Ig fusion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com