A polypeptide having both anti-oxidation and aggregation-inhibiting properties of Aβ42 and its application and gene encoding the polypeptide
An anti-oxidative and characteristic technology, applied in the field of peptides, can solve problems such as organ damage and neuron function damage, achieve major social and economic benefits, good antioxidant effects, and improve medical conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
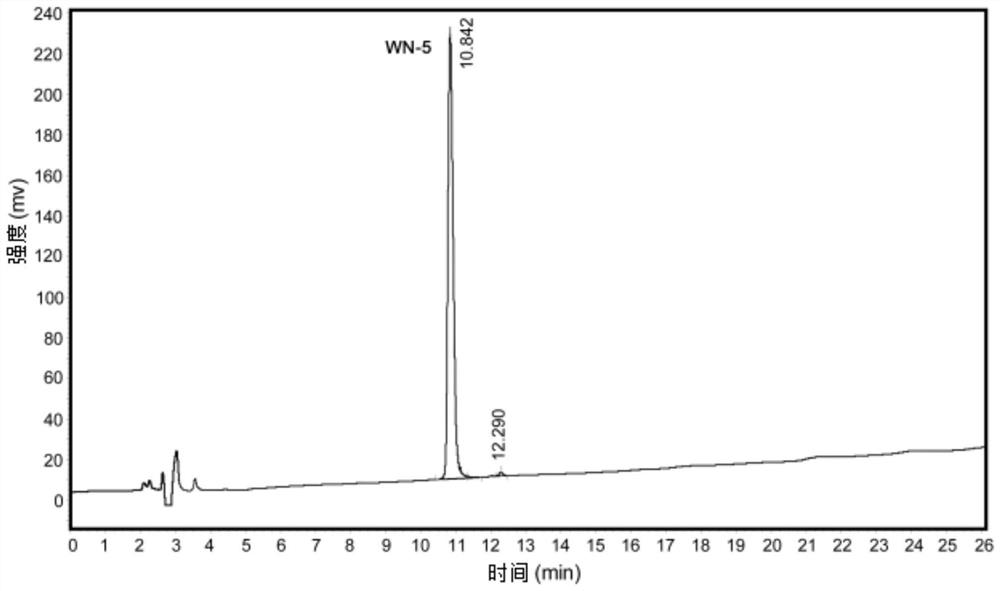
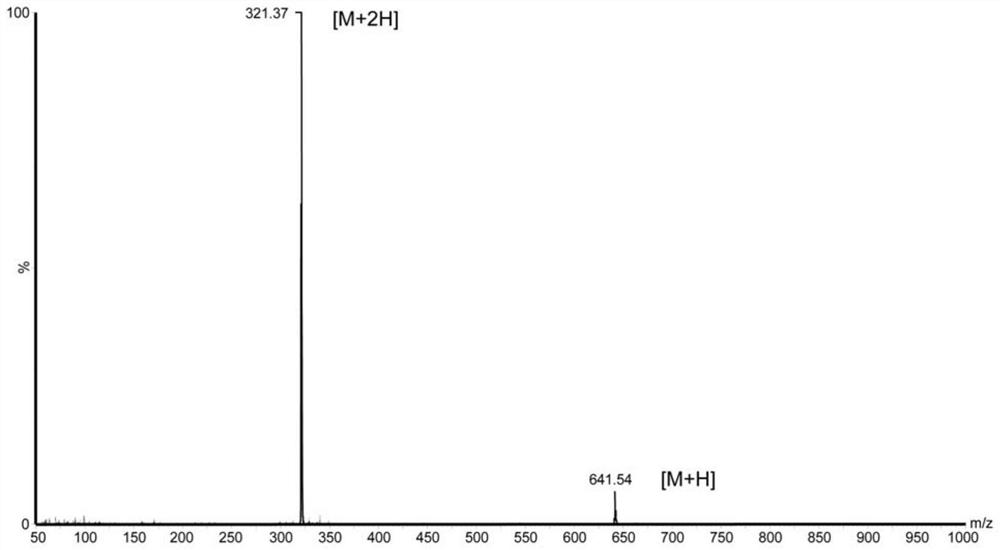
[0035] Polypeptide WN-5, which has anti-oxidation and anti-Aβ42 aggregation properties, was synthesized by peptide solid-phase synthesis, as follows:
[0036] Using the standard Fomc scheme, Wang resin was selected, and the peptide chain was extended from the C-terminal to the N-terminal one by one according to the sequence characteristics of the amino acid sequence Trp-Pro-Pro-Lys-Asn. The amount of each amino acid was 0.1mol, and 0.3mol of Fmoc was added for protection. For amino acids, HOBt is added to activate and protect the carboxyl groups of amino acids in each step of condensation, and each step of condensation is treated with 20% piperidine / N,N-dimethylformamide (DMF) solution (15ml / g) for 20min to remove the Fmoc protecting group; peptide After the side chain is synthesized, add the peptide chain containing the resin to the dichloromethane: trifluoroacetic acid mixture with a volume ratio of 99:1 to cut the peptide chain from the resin; add the peptide again to the vo...
Embodiment 2
[0039] Determination of Oxidative Free Radical Absorption Capacity of Synthetic Polypeptide WN-5
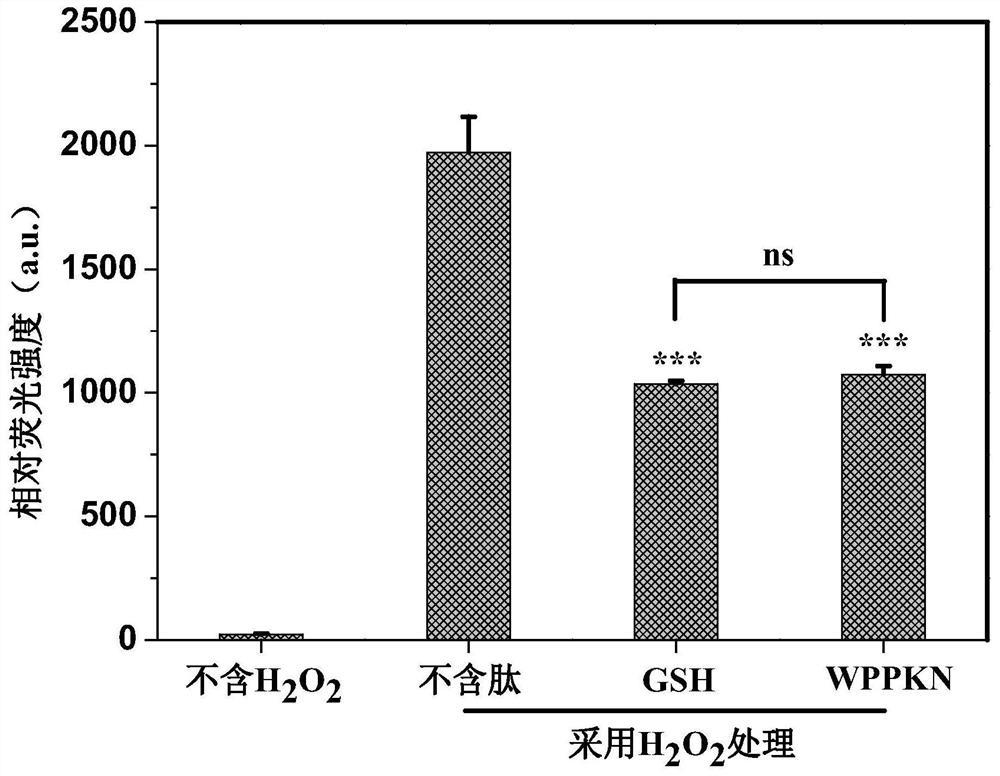
[0040] Oxygen radical absorbance capacity (ORAC) is a method for evaluating antioxidant capacity based on the hydrogen atom transfer mechanism, which is considered to be close to the antioxidant function of organisms. The ORAC reaction was carried out in a 75mM phosphate buffer solution environment at 37°C, and the synthesized polypeptide WN-5, reduced glutathione (GSH), fluorescein and AAPH free radicals were all dissolved in a 75mM phosphate buffer solution, in which The pH value of the solution was 7.4, and the final concentrations were 1.30 mM, 3.38 mM, 78 nM and 9.95 mM, respectively. Trolox was used as a standard antioxidant, and its final concentration gradient was divided into 0.5 μM, 1.0 μM, 2 μM and 4.2 μM, and GSH was used as a positive control. The synthesized polypeptide WN-5 and GSH were mixed with fluorescein respectively, and incubated at 37°C for 20 minutes, the...
Embodiment 3
[0045] Determination of DPPH·scavenging activity of synthetic polypeptide WN-5
[0046] DPPH radical scavenging activity is a method to evaluate antioxidant capacity based on electron transfer mechanism. The DPPH free radical scavenging activity reaction was carried out at 37°C. The synthesized polypeptide WN-5, ascorbic acid and DPPH were respectively dissolved in 95vol% ethanol, and the concentrations (4mM, 8mM, 12mM, 16mM and 20mM) were accurately measured with a pipette. ) 100 μL of synthetic peptide and ascorbic acid solution were put into a 96-well ELISA plate, then 100 μL of DPPH solution was added, the final concentration of DPPH was 0.2 mM, and the solution was vibrated for 30 s in a microplate reader, incubated for 15 min, and then the solution was detected at 517 nm for absorbance value, the DPPH radical scavenging activity of synthetic peptides or ascorbic acid was calculated as follows:
[0047] DPPH·clearance capacity (%)=[(A control -A sample ) / A control ]×1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com