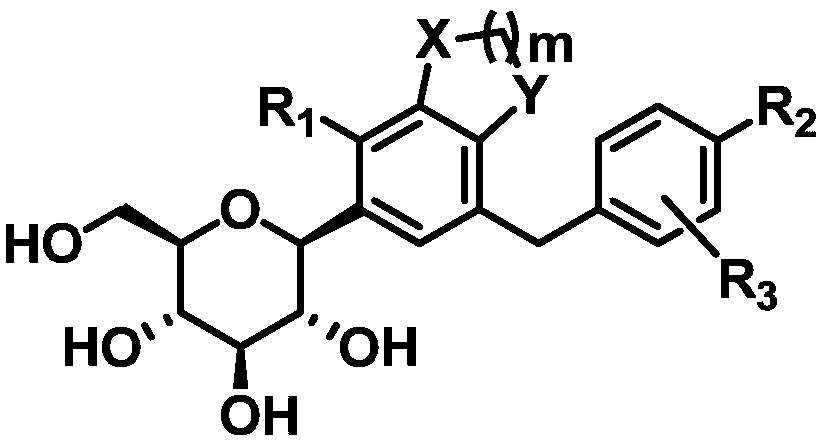
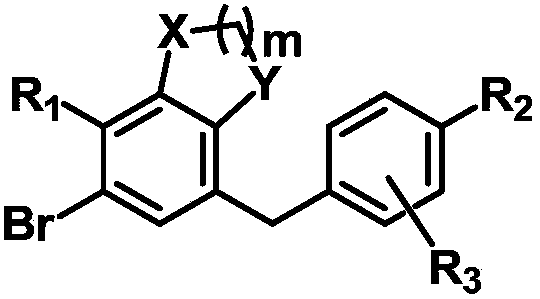
C-glucoside derivative containing fused phenyl ring or pharmaceutically acceptable salt thereof, process for preparing same, and pharmaceutical composition comprising same
一种药物学、化合物的技术,应用在C-葡糖苷衍生物或其药物学可接受的盐领域,能够解决低口服吸收性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Example 1. Preparation of (2R, 3S, 4R, 5R, 6S)-2-(hydroxymethyl)-6-(7-(4-methoxybenzyl)-4-methyl-2, 3-Dihydro-1H-inden-5-yl)tetrahydro-2H-pyran-3,4,5-triol
[0196] Step 1. Synthesis of ethyl 7-methyl-2,3-dihydro-1H-indene-4-carboxylate (1-1)
[0197]
[0198] A mixture of ethyl sorbate (25.0 mL, 170 mmol, TCI reagent) and 1-pyrrolidinyl-1-cyclopentene (24.8 mL, 170 mmol, TCI reagent) in xylene (100 mL) was stirred at reflux overnight. After the reaction was complete, the volatile solvent was evaporated under reduced pressure. EtOAc was added to the resulting mixture. The organic layer was washed with brine, after which the resulting product was dissolved in anhydrous MgSO 4 Dry over, filter and concentrate in vacuo. The crude compound was used in the following step without additional purification. Will S 8 (5.45 g, 170 mmol) was added to the crude compound. The reaction mixture was stirred at 250°C for 2 hours. After the reaction was complete, the resul...
Embodiment 2 and 3
[0225] The target compounds of Examples 2 and 3 were obtained by the method shown in Example 1.
Embodiment 2
[0226] Example 2. Preparation of (2S,3R,4R,5S,6R)-2-(7-(4-ethoxybenzyl)-4-methyl-2,3-dihydro-1H- Inden-5-yl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
[0227]
[0228] 1 H NMR (400MHz, CD 3 OD); δ7.10(s, 1H), 7.03(d, J=8.4Hz, 2H), 6.76(d, J=8.8Hz, 2H), 4.45(d, J=8.8Hz, 1H), 3.97( q, J=6.8Hz, 2H) 3.88-3.84(m, 3H), 3.67-3.48(m4H), 3.40-3.38(m, 2H), 2.84(t, J=7.6Hz, 2H), 2.73(t, J=7.6Hz, 2H), 2.28(s, 3H), 02.02-1.97(m, 2H), 1.35(t, J=6.8Hz, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com