Novel anthracene luminescent material, preparation method, and applications thereof
A luminescent material, anthracene technology, applied in the field of new anthracene luminescent materials and their preparation, can solve the problem of quantum efficiency less than 1%, and achieve the effect of improving luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of 2,3,6,7,9,10-hexa-bromoanthracene
[0054] Under the condition of nitrogen protection, 1 part of anthracene was dissolved in dichloromethane, and iron powder (the molar ratio of iron powder:anthracene was 1:1~10), bromine water (the molar ratio of bromine water:anthracene was 1:1) ~10) respectively add to a single-neck bottle, stir vigorously at room temperature for 6h, filter to obtain insoluble matter, and further wash with dilute hydrochloric acid, purified water and acetone to obtain 2,3,6,7,9,10-hexa-bromoanthracene (shown by 1 in formula (1)), its synthetic route is shown in formula (1), and the yield of 2,3,6,7,9,10-hexa-bromoanthracene is about 70%.
[0055]
Embodiment 2
[0057] Under the condition of nitrogen protection, 1 part of 9-anthracene was dissolved in dichloromethane, and iron powder (the molar ratio of iron powder: 9-bromoanthracene was 1:1~10), bromine water (bromine water: 9-bromine The molar ratio of anthracene is 1:1~10) were added into single-neck flasks, stirred vigorously at room temperature for 6h, filtered to obtain insoluble matter, and further washed with dilute hydrochloric acid, purified water and acetone to obtain mixtures 2, 3, 6, 7,9,10-hexa-bromoanthracene (shown by 1 in formula (2)) and 2,3,6,7,9-penta-bromoanthracene (shown by 1' in formula (2)), its synthetic route As shown in formula (2), the yield of 2,3,6,7,9,10-hexa-bromoanthracene is about 65%.
[0058]
Embodiment 3
[0059] Example 3 Preparation of 2,3,6,7,9,10-hexa-(4-methoxyphenyl)anthracene
[0060] 1. Under the condition of nitrogen protection, the 2,3,6,7,9,10-hexa-bromoanthracene and 4-methoxyphenyl borate (molar ratio is 1:7) in step 1, Add tetrakis(triphenylphosphine)palladium and potassium carbonate into a single-necked bottle, then 10-20mL of toluene and ethanol, stir vigorously, keep the temperature at 90°C, reflux for 24h, extract, wash and filter to obtain the initial product, and go through column chromatography Chromatography or recrystallization, obtain 2,3,6,7,9,10-hexa-(4-methoxyphenyl) anthracene, its molecular structure is as shown in 2a in formula (3), and its synthetic route is as formula ( 3), the yield is about 60%.
[0061]
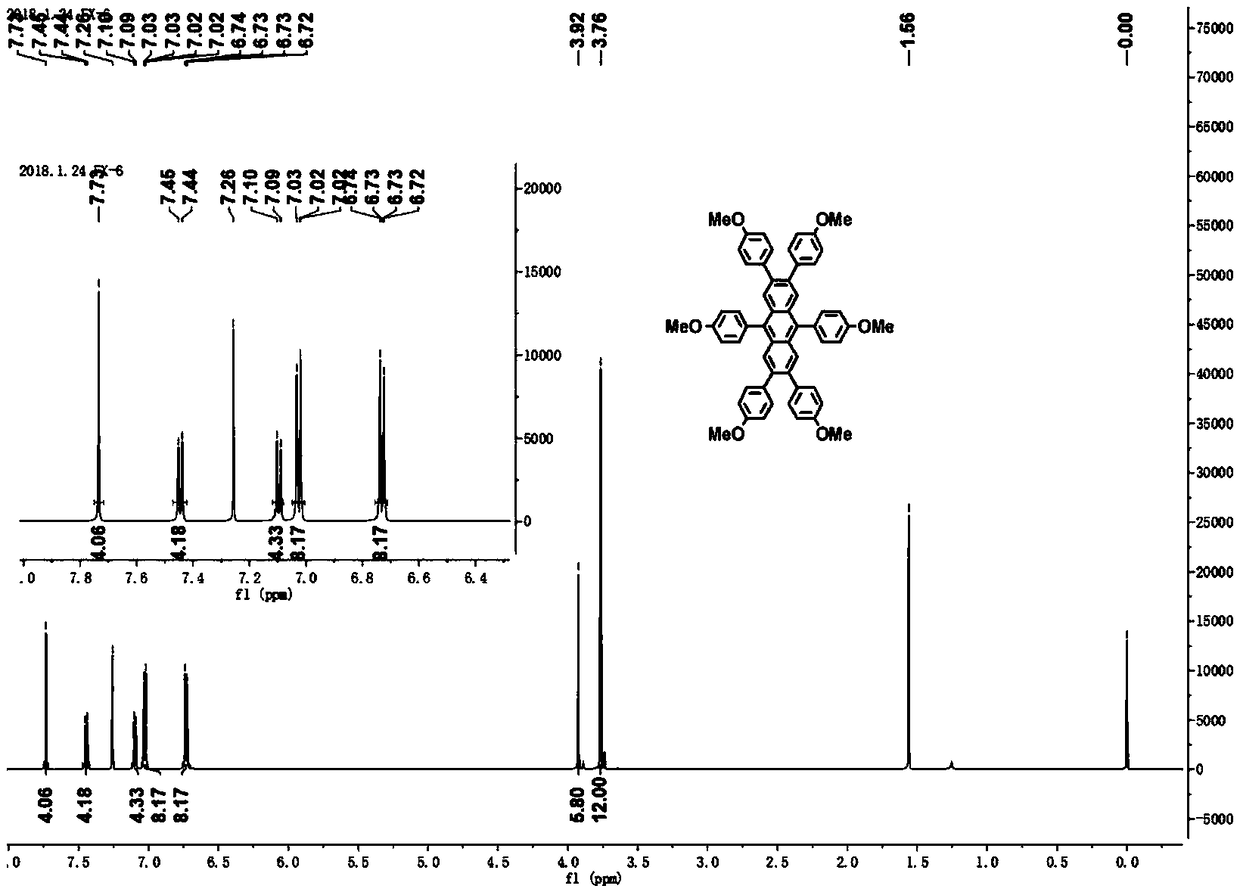
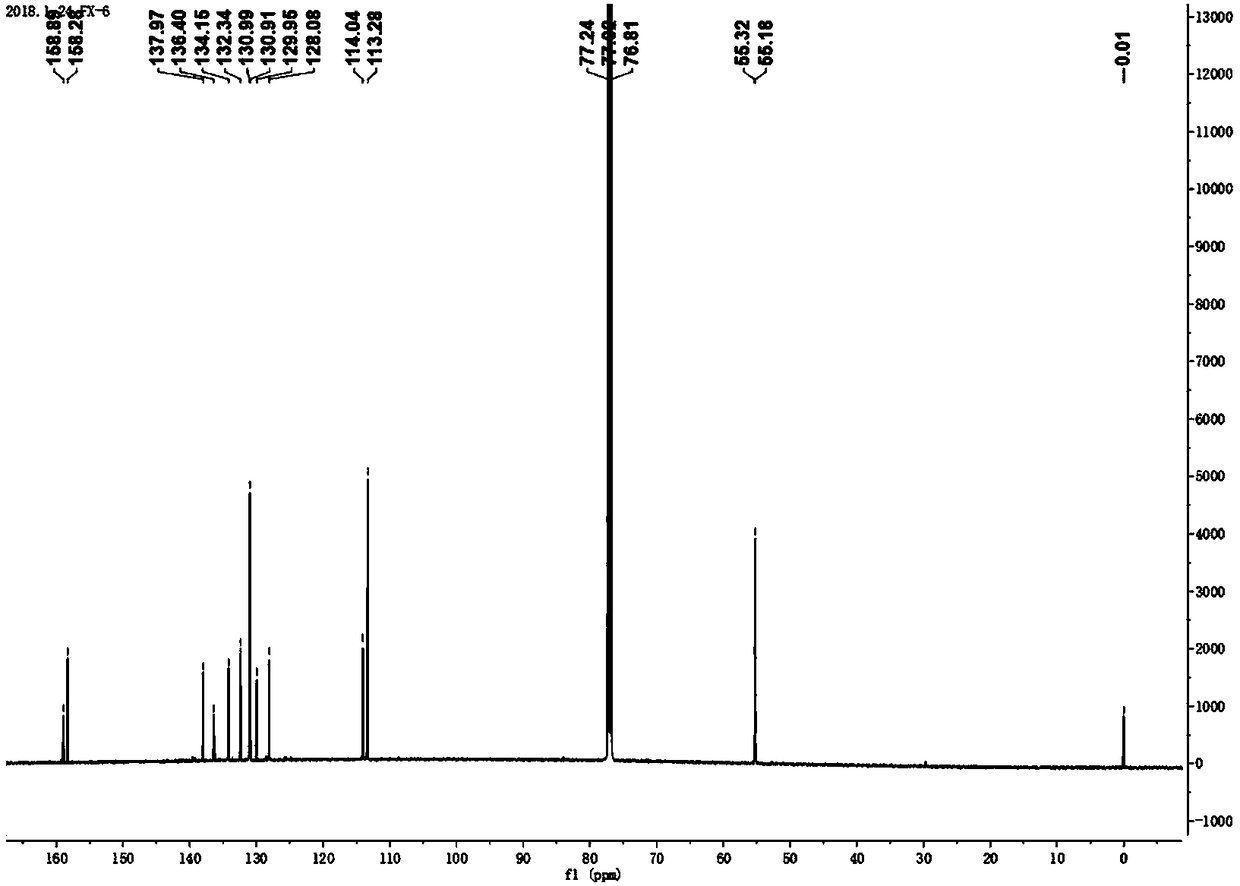
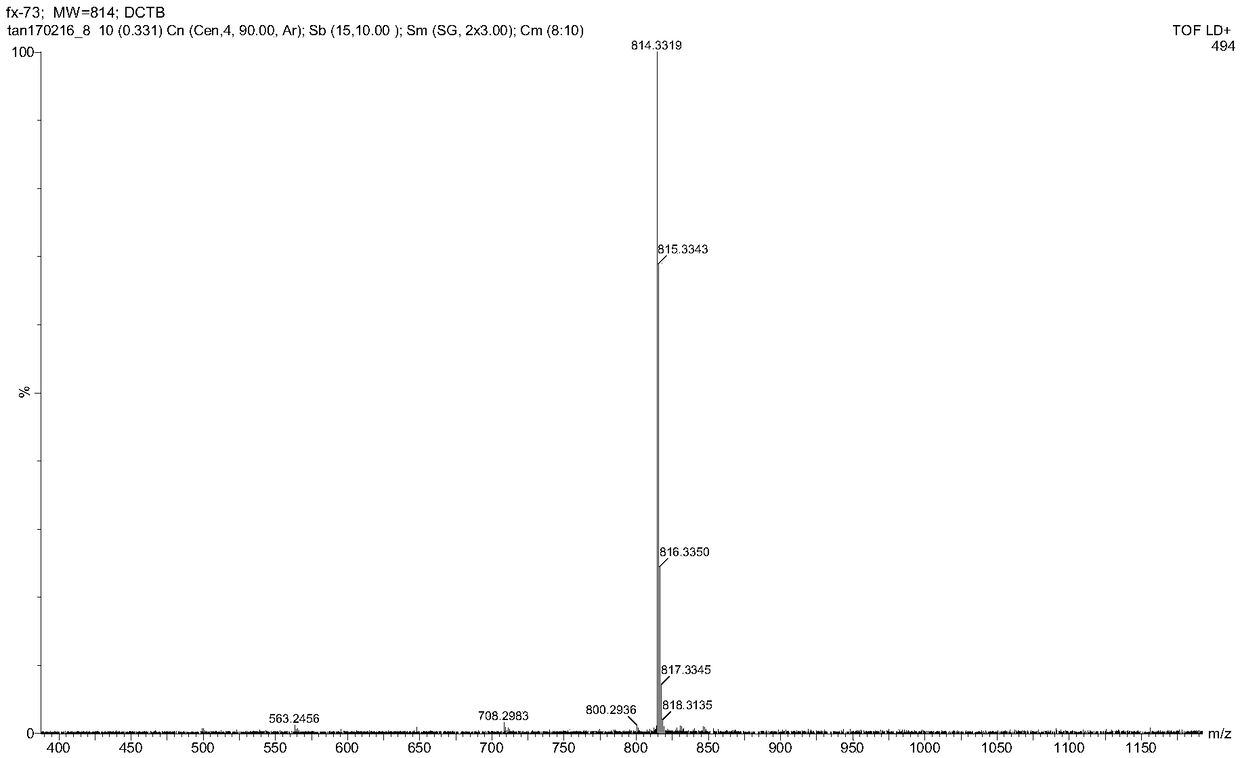
[0062] figure 1 For the 2,3,6,7,9,10-sixa-(4-methoxyphenyl)-anthracene obtained in this example 1 H CNMR diagram. figure 2 For the 2,3,6,7,9,10-sixa-(4-methoxyphenyl)-anthracene obtained in this example 13 C NMR image. image 3 In or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com