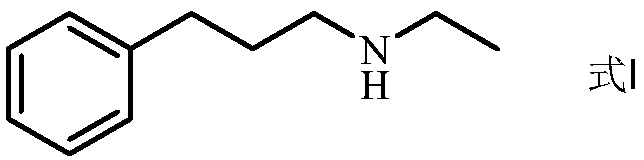
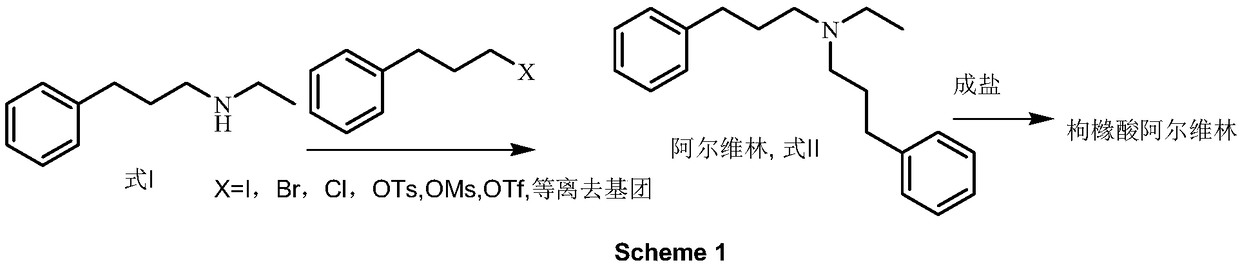
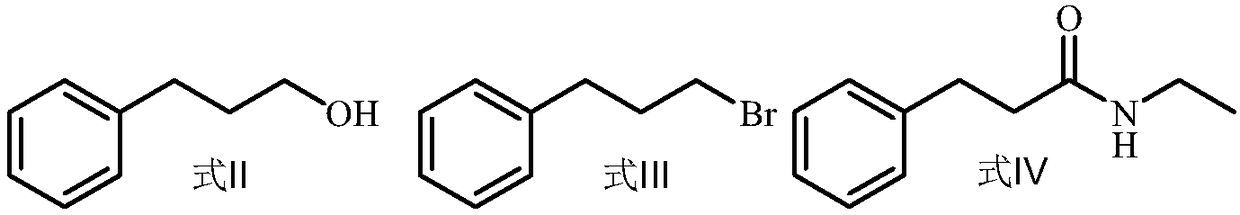
Preparation method of N-ethyl-3-phenylpropylamine
A technology of phenylpropylamine and phenylpropanal, applied in the field of compound preparation, can solve the problems of high equipment requirements, violent reaction of reagents, harsh reaction conditions, etc., achieves low requirements for equipment and operators, avoids tertiary amines and quaternary amines The effect of ammonium salt by-product and post-processing is simple and inexpensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Under nitrogen protection, 20 mmol ethylamine hydrochloride was dissolved in 20 mL methanol, and stirred for 10 min. At -40°C, 20 mmol of 3-phenylpropanal (Formula V) was added dropwise. The progress of the reaction was monitored by gas chromatography. After reaching the end of the reaction, slowly add 5 mmol of NaBH at -40°C 4 , continue to monitor the progress of the reaction by gas chromatography. After reaching the end of the reaction, the temperature was raised to -20°C, and 20 mL of methanol-water (V 甲醇 :V 水 =10:90) solution to quench the reaction, and continue to stir, and monitor the progress of the reaction by gas chromatography. After reaching the end point of the reaction, the reaction was terminated.
Embodiment 2
[0090] Take the reaction solution of Example 1, filter it, evaporate the solvent to dryness under reduced pressure, and adjust the pH=8. The aqueous layer was extracted twice with ethyl acetate, the organic layers were combined, extracted once with water and saturated brine, and the organic layer was collected; dried, filtered, and evaporated to dryness under reduced pressure to obtain the target compound N-ethyl-3-benzene Propylamine (formula I, yield 72%, its purity 85% detected by gas chromatography).
Embodiment 3
[0092] Under the protection of nitrogen, 20 mmol of ethylamine hydrochloride was dissolved in 20 mL of methanol, and after slowly adding 2 mmol of LiOH, the mixture was stirred for 10 min. At -40°C, 20 mmol of 3-phenylpropanal (Formula V) was added dropwise. The progress of the reaction was monitored by gas chromatography. After reaching the end of the reaction, slowly add 5 mmol of NaBH at -40°C 4 , continue to monitor the progress of the reaction by gas chromatography. After reaching the end of the reaction, the temperature was raised to -20°C, and 20 mL of methanol-water (V 甲醇 :V 水 =10:90) solution to quench the reaction, and continue to stir, and monitor the progress of the reaction by gas chromatography. After reaching the end of the reaction, filter, evaporate the solvent under reduced pressure, and adjust the pH to 8. The aqueous layer was extracted twice with ethyl acetate, the organic layers were combined, extracted once with water and saturated brine, and the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com