Application of celecoxib derivative OSU-03012 in preparation of anti-tubercle bacillus drugs
A technology for celecoxib and anti-tuberculosis bacteria, applied in antibacterial drugs, drug combinations, respiratory diseases, etc., can solve the unreported OSU-03012 and other problems, and achieve good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
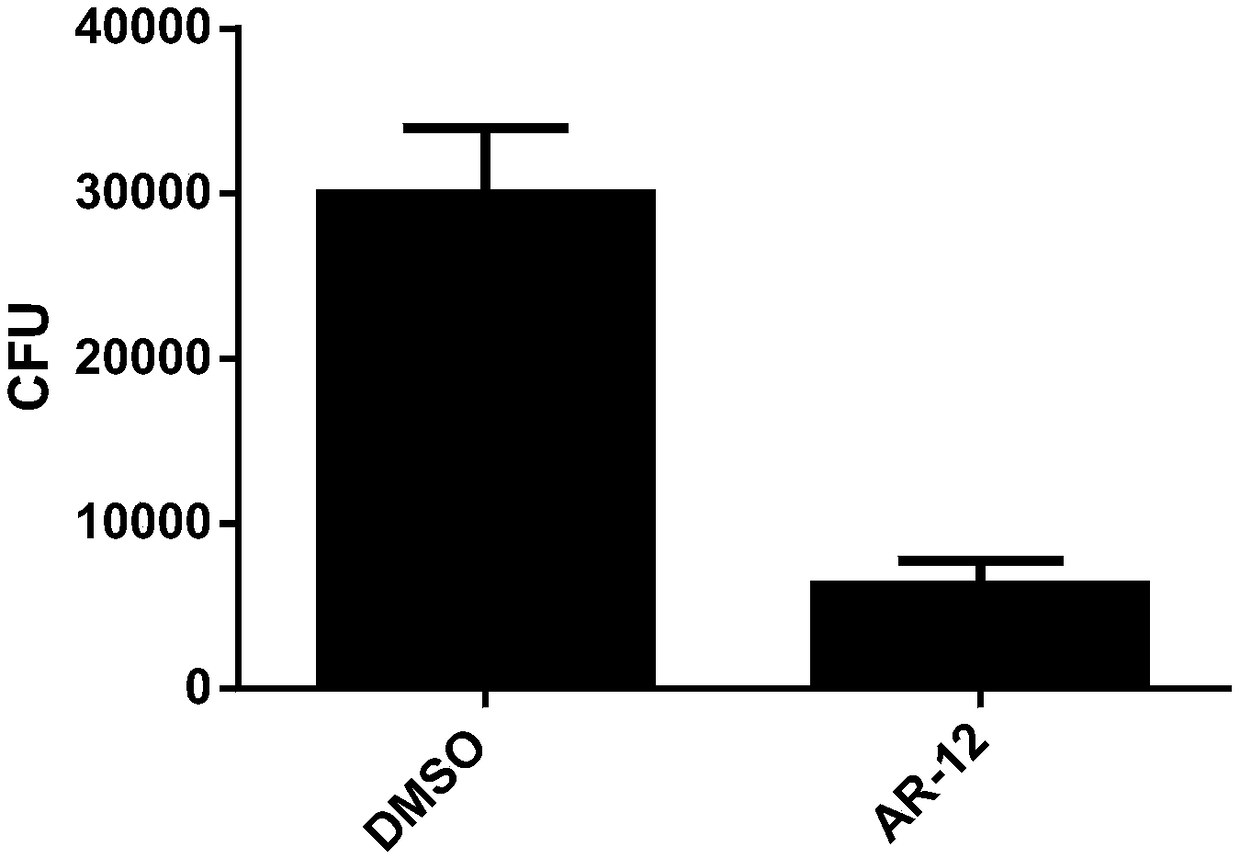
[0027] The C57BL / 6 mouse peritoneal primary macrophages were divided into 1*10 5 Each cell / well was inoculated in a 48-well cell culture plate. After about 2 hours for the cells to adhere to the wall, the complete medium 1640 was removed, and then fresh complete medium was added to culture overnight. The next day, 1 h before infection, replace with fresh 1640 containing 10% serum without double antibody, and add AR-12 at a final concentration of 1uM in the cell culture supernatant; use DMSO as a control well, place the cells in After culturing in the incubator for 1 h, the tubercle bacillus (H37Rv) was infected at a dose of MOI=5. After 2-3 hours of infection, discard the supernatant, then culture the cells with amikacin-containing medium for 2 hours, discard the supernatant and replace with 1640 containing 10% serum without double antibody and continue at 37°C for 5 %CO 2 Cells were cultured in the incubator for 24 h. Discard the supernatant and wash the cells with PBS, th...
Embodiment 2
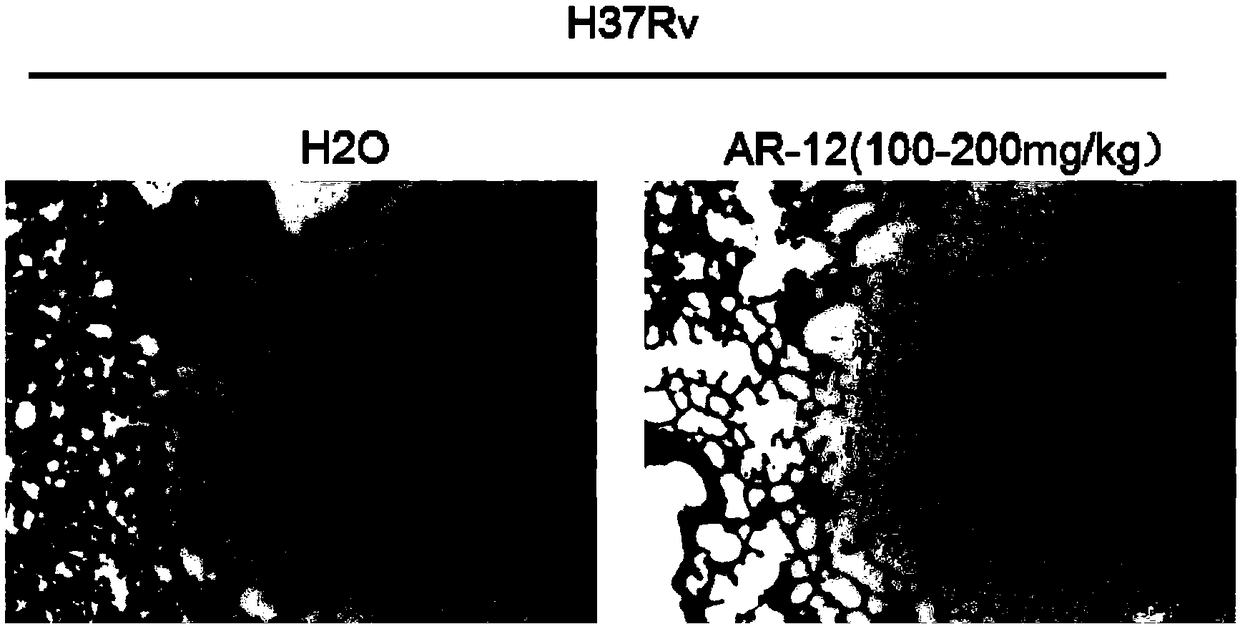
[0030] The C57BL / 6 mice were divided into two groups, 6 in each group, and infected with Mycobacterium tuberculosis (H37Rv) by intraperitoneal injection, the dose was 2*10 6 Bacteria / mouse, 1 week after infection, orally administered 25 mg / kg and PBS as a control, and administered continuously for 3 weeks. The mice were sacrificed by dislocation of the cervical spine, and one lobe of the lung was taken out, fixed with 4% paraformaldehyde, paraffin sectioned, and H&E staining was performed to observe the pathological damage of the lung.
[0031] see figure 2 , given AR-12 at a dose of 100-200 mg / kg, after three weeks of oral administration, the pathological damage of the lungs of mice was significantly reduced; therefore, the experimental results of this example show that AR-12 can effectively reduce the damage of the lungs of mice. Pathological damage.
Embodiment 3
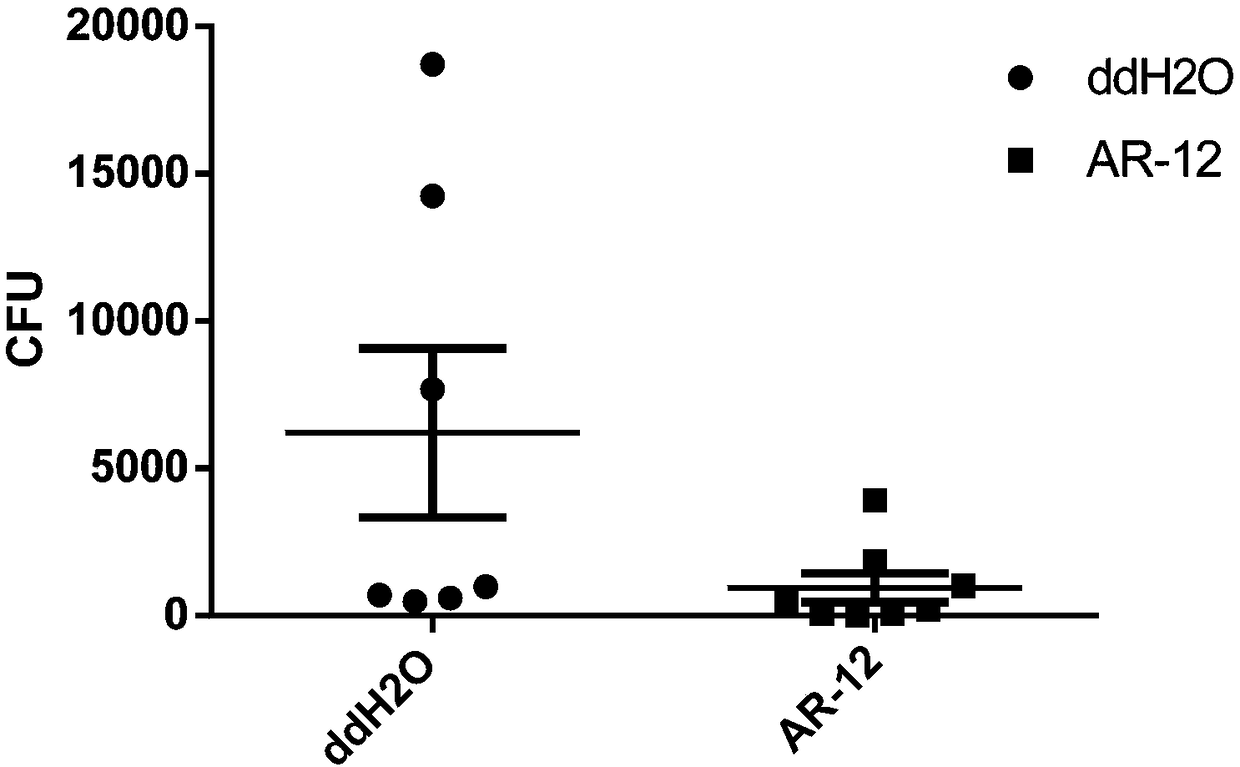
[0033] Take one-third of the mouse lung tissue administered three weeks after infection in Example 2, and grind it with 1 ml of PBS containing 1% triton-100, serially dilute, and take 10 -3 、10 -4Spread 100ml of tissue suspension evenly on Middle Brook 7H10 agar culture plate containing amphotericin B, then place it in a 37°C incubator for 2-3 weeks, and count the colonies. The results are as follows: image 3 shown.
[0034] The experimental results of this example show that AR-12 significantly reduces the bacterial load of Mycobacterium tuberculosis in the lungs of mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com