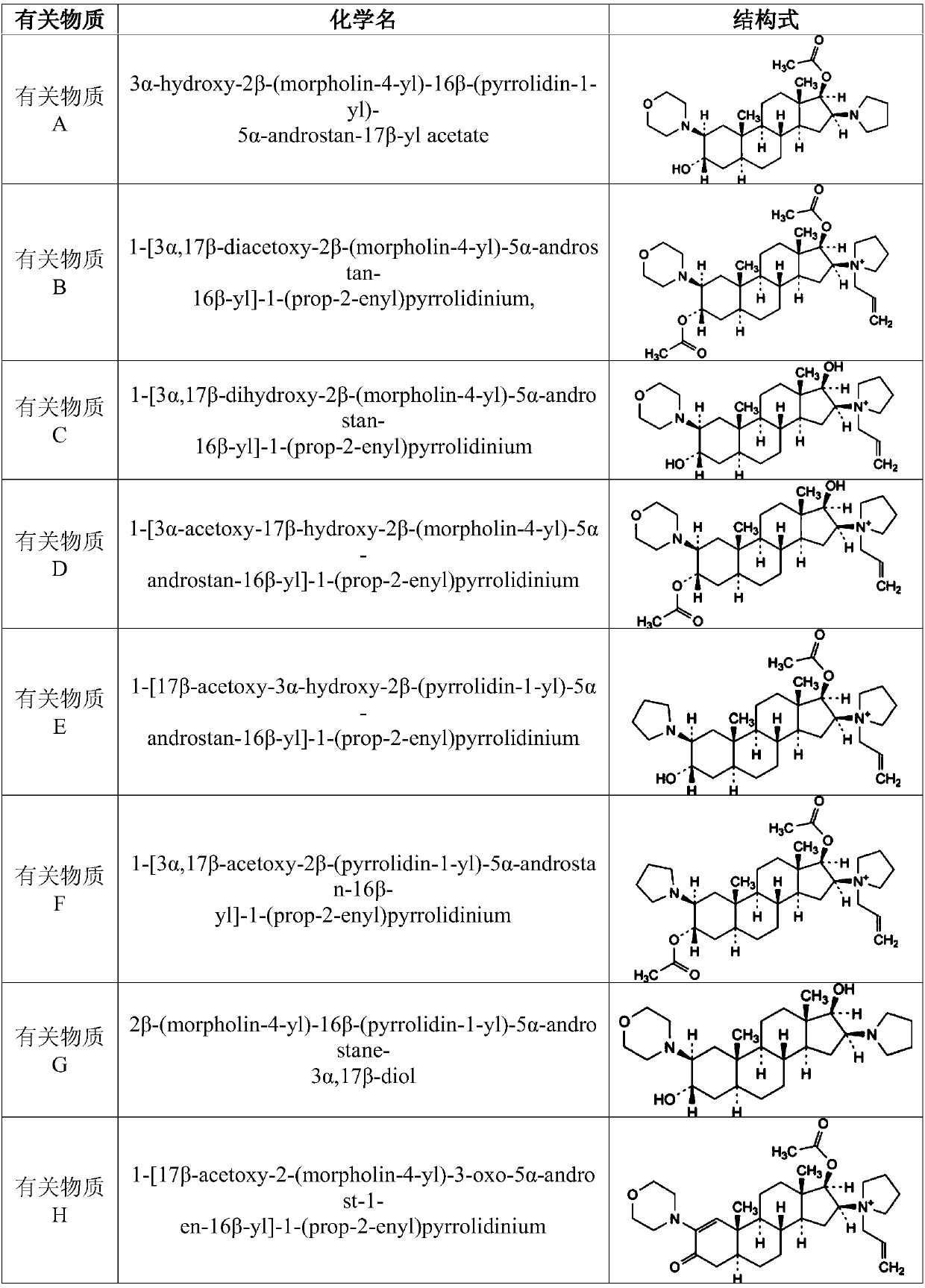
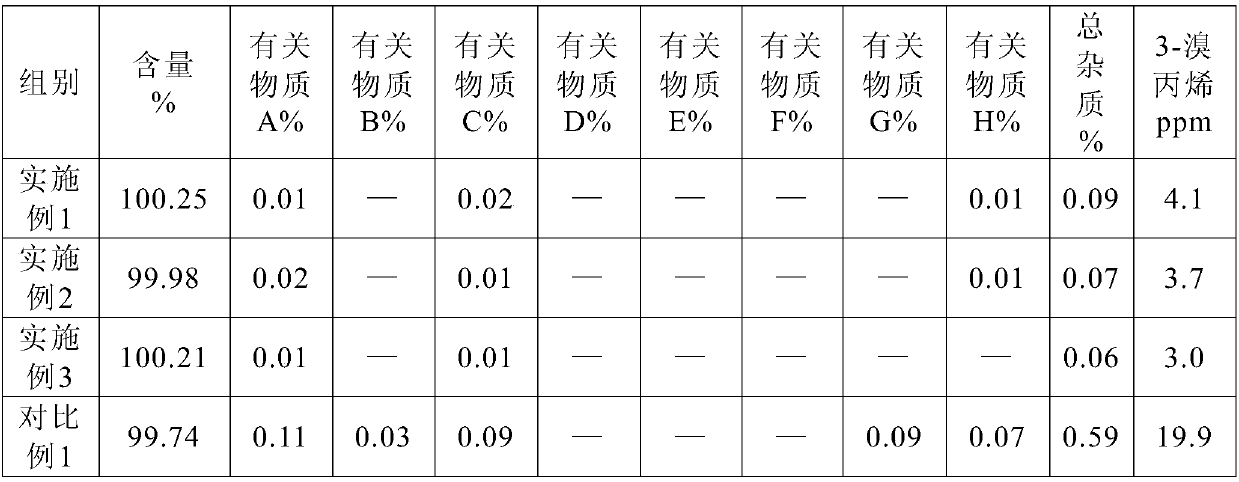
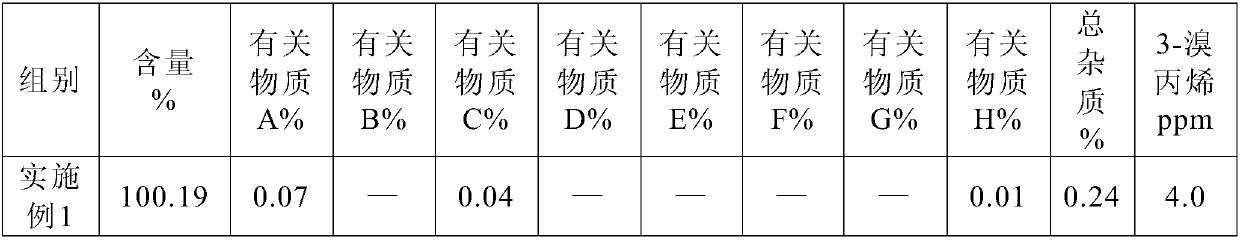
Preparation method and pharmaceutical composition of rocuronium bromide
A technology of raw materials and crude products, which is applied in the field of preparation method of rocuronium bromide and its pharmaceutical composition, can solve the problems of dose dependence, fast action and slow onset of rocuronium bromide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of rocuronium bromide raw material is:
[0025] Take 100g of crude rocuronium bromide, first add 550ml of chloroform, stir to dissolve, cool down to 5-10°C, add 250ml of deionized water, stir evenly, and introduce CO 2 , CO 2 The flow rate was 15ml / min, 300ml of diethyl ether was added after 40min, allowed to stand, filtered, the filter cake was washed with diethyl ether, freeze-dried to obtain 81.2g of rocuronium bromide raw material.
Embodiment 2
[0027] The preparation method of rocuronium bromide raw material is:
[0028] Take 100g of crude rocuronium bromide, first add 850ml of chloroform, stir to dissolve, cool down to 5-10°C, add 400ml of deionized water, stir evenly, and introduce CO 2 , CO 2 The flow rate was 25ml / min, 500ml of diethyl ether was added after 20min, allowed to stand, filtered, the filter cake was washed with diethyl ether, freeze-dried to obtain 81.9g of rocuronium bromide raw material.
Embodiment 3
[0030] The preparation method of rocuronium bromide raw material is:
[0031] Take 100g of crude rocuronium bromide, first add 700ml of chloroform, stir to dissolve, cool down to 5-10°C, add 420ml of deionized water, stir evenly, and introduce CO 2 , CO 2 The flow rate was 20ml / min, 400ml of diethyl ether was added after 30min, allowed to stand, filtered, the filter cake was washed with diethyl ether, freeze-dried to obtain 83.8g of rocuronium bromide raw material. Comparative example 1:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com