Recombinant bat adeno-associated virus vector and applications thereof
A viral vector, recombinant vector technology, applied in the direction of virus/phage, vector, virus, etc., can solve the problem of not using bat AAV and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The construction of embodiment 1 recombinant vector
[0043] (1) Amplify the full-length bat AAV capsid gene (GenBank, No.GU226971) from the AAV feces samples of Yunnan bats collected in 2007, and obtain the 2.5kb PCR product 07YN. The primers are as follows:
[0044] Bt-CAP5`:5`-CCC AAGCTT CGGGGGAATCTGACTCCGTGAACTTCGCCGAGA-3' (Seq ID NO. 6).
[0045] Bt-CAP3`:5`-GGA AGATCT CGCAGAGACCAAAGTTCAACTGAAACGA-3' (Seq ID NO. 7).
[0046] Digest the above PCR product with HindIII / BglII, recover a 2.5kb fragment, and at the same time digest UF1-AAV8 with HindIII / NotI, recover a 6.0kb fragment, connect the two fragments with T4 DNA ligase, and construct the plasmid UF1-07YN .
[0047] (2) In order to perform PCR amplification on more bat DNA samples, a degenerate primer dCAP3` was designed, together with primer Bt-CAP5`, to amplify bat AAV full-length coats from bat feces samples obtained in Yunnan and Hubei respectively. Shell gene, two 2.5kb PCR products 09YN and 10HB were ...
Embodiment 2
[0055] Example 2 Capability verification of AAV plasmid packaging wild-type bat AAV
[0056] The constructed AAV plasmids (UF1-07YN, UF1-09YN, UF1-1285, UF1-10HB) and the positive control UF1-AAV8 were packaged with the adenovirus 5 helper packaging plasmid phelper by calcium phosphate co-precipitation method to package the recombinant virus. The experimental steps, reagents, conditions and methods are as follows:
[0057] The standard plasmid described in the experiment is UF1-AAV8, which and the recombinant wild-type virus both carry the REP gene, so the probe targeting the REP gene can be used for dot hybridization experiments to determine the titer of the recombinant wild-type virus. The positive and negative controls are the supernatant obtained by transfecting 293 cells with the plasmid UF1-AAV8. The former is added with the adenovirus phelper plasmid necessary for packaging AAV, and the latter is not added with the phelper plasmid. These two controls are mainly set to e...
Embodiment 3
[0059] Example 3 Capability verification of AAV plasmid packaging recombinant bat AAV
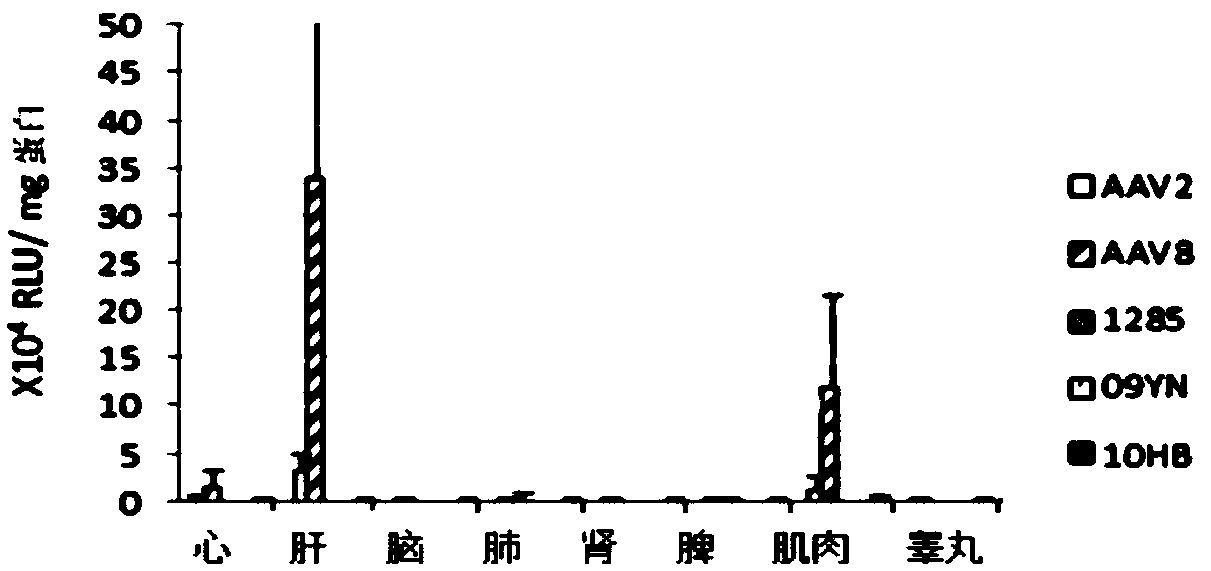
[0060] Adenovirus phelper plasmid, recombinant bat AAV plasmid AAV2-07YN, AAV2-09YN, AAV2-1285, AAV2-10HB and plasmid AAV-luc1 (from University of North Carolina, USA) carrying AAV2 ITR and luciferase reporter gene were used The recombinant virus was packaged by calcium phosphate precipitation method. The experimental steps, reagents, conditions, and methods are as follows: AAV2 in the plasmid name represents the REP gene of AAV2 used, and AAV2 in the figure represents the recombinant AAV2 virus produced by packaging the AAV2 capsid protein gene.
[0061] The 293 cells were subcultured into a 6-well plate at a ratio of 1:3. After 24 hours, the cell density was about 70-80%. Adenovirus phelper plasmids, AAV packaging plasmids (including AAV2-07YN, AAV2-09YN, AAV2-1285, AAV2-10HB), AAV-luc plasmids carrying AAV2ITR and luciferase reporter gene were added in 300 μl 0.25M CaCl at a mass ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com