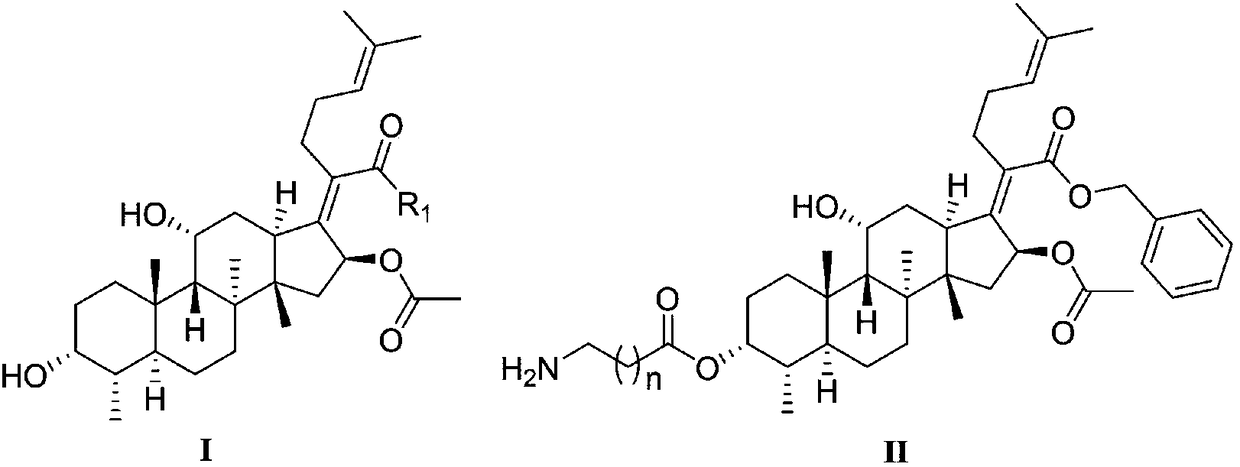
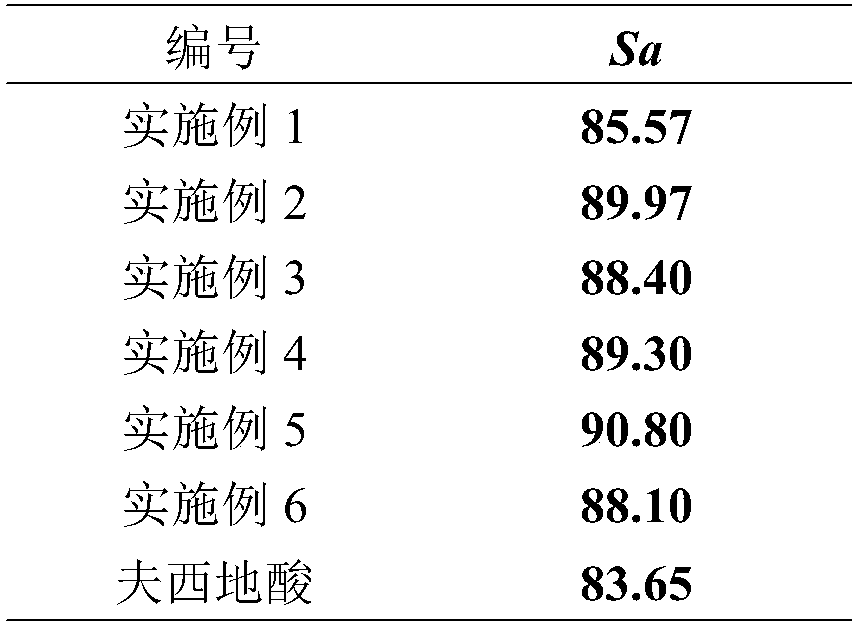
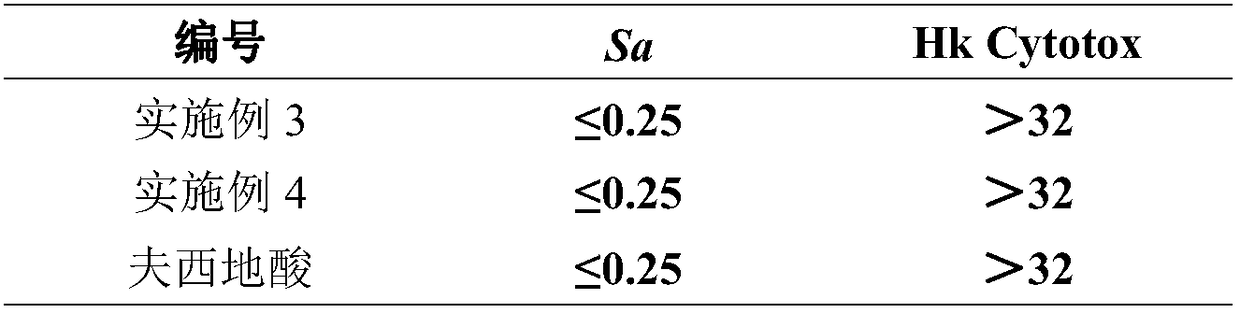
Novel fusidic acid derivative as well as synthesis preparation method and application thereof
A technology of fusidic acid and derivatives, which is applied in the field of fusidic acid derivatives, can solve the problems of low resistance barrier and easy occurrence of drug resistance, and achieve the effect of improving antibacterial activity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 21-Fusidic acid (N,N'-dicyclohexyl) carbodiimide ester
[0054] Take a 50mL eggplant-shaped bottle, dissolve fusidic acid (150mg, 0.29mmol) in anhydrous dichloromethane (20mL), stir and add N,N'-dicyclohexylcarbodiimide (180mg, 0.87mmol), DMAP (105mg, 0.86mmol), react at room temperature for 9-11 hours. Wash with 10% hydrochloric acid to acidity successively, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, silica gel column chromatography (V 氯仿 :V 甲醇 =160:1-140:1), a white solid (175mg, 83.2%) was obtained. 1 H-NMR (CDCl 3,400MHz)δ:5.56(d,J=8.72Hz,1H,16-H),5.00(t,J=5.66Hz,1H,24-H),4.29(s,1H,11-OH),3.67( d,J=2.14Hz,1H,3-OH),3.58-3.63(m,1H,3-H),2.94-3.02(m,J=12.24Hz,1H,13-H),2.50-2.63(m ,1H,-CH-),2.21(m,1H,-NH-),1.90-1.91(m,2H,2×22-H),1.87-1.89(m,2H,12-H and 15-H) ,1.74-1.84(m,4H,1-H,5-H and 2×23-H),1.68(s,3H,OCOCH 3 ),1.65-1.67(m,2H,2-H and 12-H),1.58-1.61(m,2H,2-H and 7-H),1.53(s...
Embodiment 2
[0056] 21-Fusidic acid [1-ethyl-(3-dimethylaminopropyl)] carbodiimide ester
[0057] Referring to the synthesis method of 21-fusidic acid (N,N'-dicyclohexyl) carbodiimide ester, fusidic acid and 1-ethyl-(3-dimethylaminopropyl) carbonyl diimide The imine was reacted to give a yellow solid (126 mg, 52.3%). 1 H-NMR (CDCl 3 ,400MHz)δ:9.14(s,1H,-NH-),5.93(d,J=8.83Hz,1H,16-H),5.06(t,J=6.92Hz,1H,24-H),4.35( s,1H,11-H),3.75(d,J=2.30Hz,1H,3-OH),3.53-3.61(td,J=4.79,10.96Hz,2H,-CH 2 -),3.24-3.38(m,2H,-CH 2 -), 3.03(d, J=11.49Hz, 1H, 13-H), 2.68-2.75(m, 2H, -CH 2 -),2.27(s,6H,2×-CH 3 ),2.18-2.22(m,2H,-CH 2 -),2.08-2.15(m,3H,12-H and 2×22-H),2.00-2.05(m,3H,15-H and2×23-H),1.95-1.96(m,2H,1- H and 5-H),1.91(s,3H,OCOCH 3 ),1.82-1.86(m,2H,2-H and12-H),1.72-1.78(m,2H,2-H,7-H),1.67(s,3H,27-CH 3 ),1.60(s,3H,26-CH 3 ),1.49-1.53(m,3H,1-H,6-H and 9-H),1.38(s,3H,-CH 3 ),1.26(s,3H,30-CH 3 ), 1.17(t, J=7.25Hz, 1H, -CH 2 -),1.04-1.13(m,2H,6-H and 7-H),0.98(s,3H,19-CH 3 ),0.93(s,3H,18-CH 3...
Embodiment 3
[0059] 21-Fusidic acid (6-chloro-benzotriazole-1) ester
[0060] Take a 50mL eggplant-shaped bottle, dissolve fusidic acid (220mg, 0.42mmol) in anhydrous dichloromethane (20mL), stir and add 6-chloro-1-hydroxybenzotriazole (267mg, 1.30mmol), EDCI (245mg, 1.28mmol), react at room temperature for 4-6 hours. Diluted with dichloromethane (20mL), washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporated to remove solvent under reduced pressure, silica gel column chromatography (V 氯仿 :V 甲醇 =160:1-140:1), a white solid (233 mg, 85.8%) was obtained. 1 H-NMR (CDCl 3 ,400MHz) δ:7.99(d,J=9.36Hz,1H,Ar-H),7.40(d,J=1.76Hz,1H,Ar-H),7.38(d,J=1.93Hz,1H,Ar-H) H), 5.93(d, J=8.42Hz, 1H, 16-H), 5.23(t, J=7.20Hz, 1H, 24-H), 4.42(s, 1H, 11-H), 3.77(d, J=2.3Hz, 1H, 3-OH), 3.23(d, J=10.58Hz, 1H, 13-H), 2.76-2.82(m, 1H, 22-H), 2.62-2.69(m, 1H, 22 -H),2.37-2.44(m,1H,12-H),2.29-2.34(m,2H,2×23-H),2.12-2.22(m,2H,1-H and5-H),2.09( s,1H,15-H),1.84-1.99(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com