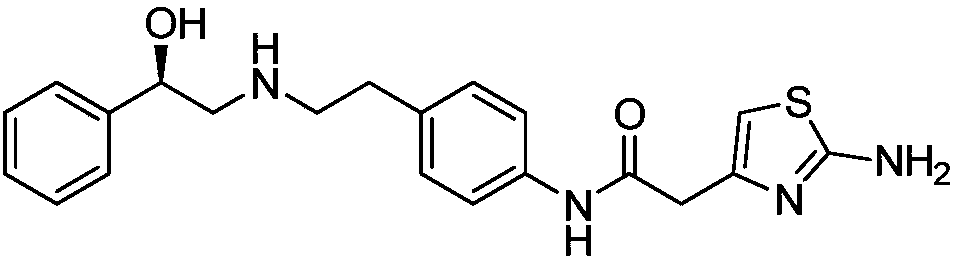
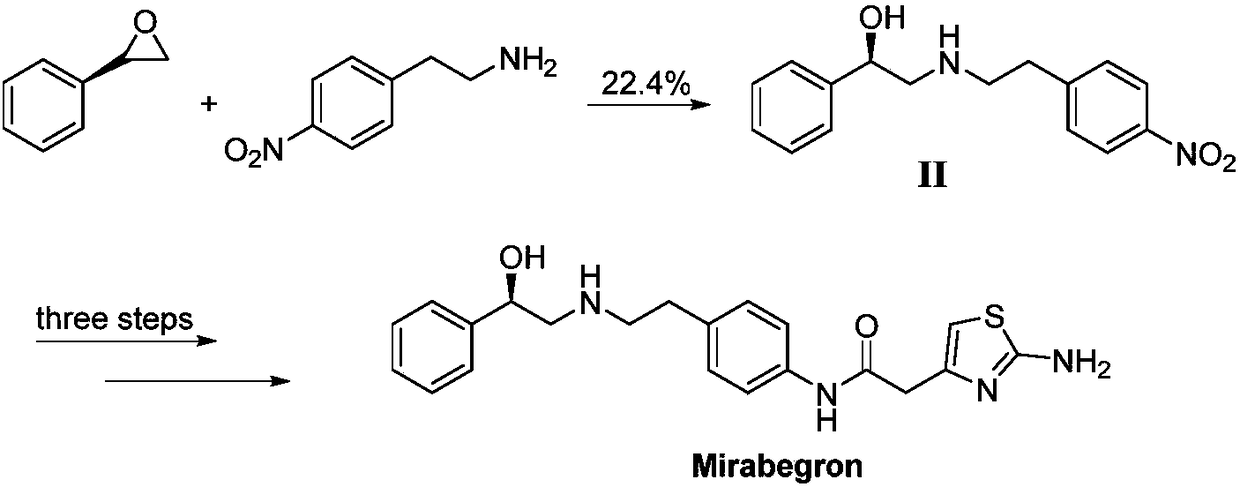
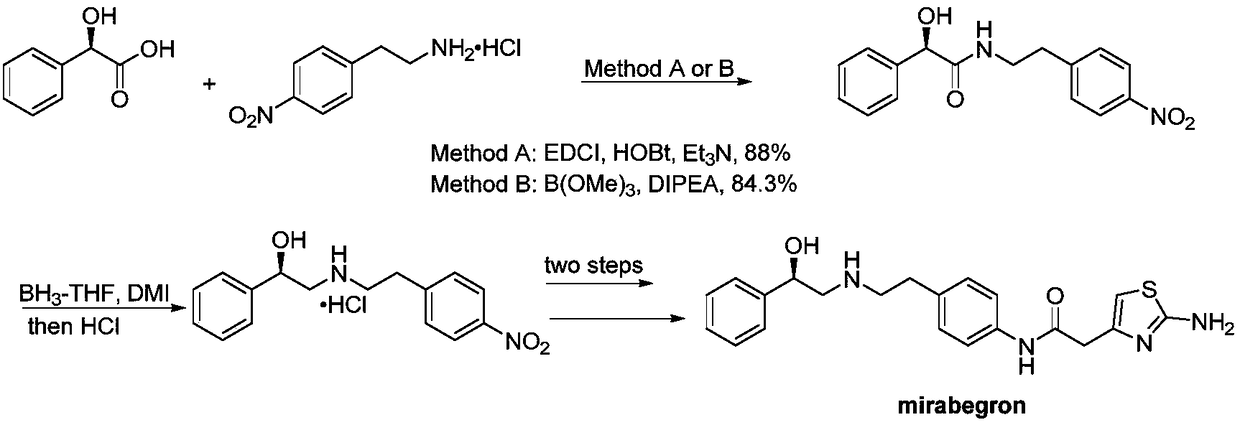
Synthesis of mirabegron intermediate (R)-2-(4-nitrophenethylamino)-1-phenylethanol hydrochloride
A technology of nitrophenethylamino and phenylethanol, which is applied in the field of drug synthesis, can solve the problems of many condensation by-products, cumbersome post-processing steps, and high price, and achieve the benefits of large-scale industrial production and the feasibility of industrial operation Great effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Intermediate I: Add 20g of α-bromoacetophenone, 16.6g of 4-nitrophenethylamine, 48.9g of cesium carbonate, and 150mL of acetonitrile into a 250mL three-neck flask, react at 70°C for 5h, cool to room temperature, filter, and vacuum Concentrate the solvent to obtain an oil, add 50mL of petroleum ether to crystallize for 12h, filter, wash with 20mL of petroleum ether, and dry to obtain 20.7g of white solid, Intermediate I, with a yield of 73% and a purity of 99.31%.
[0034] Preparation of intermediate II hydrochloride: nitrogen protection, -5 ° C, BH 3 -THF (1M, 70mL) was added dropwise to CBS (1M, 3.6mL,) for 30 minutes, and stirred for 60 minutes after the addition, and intermediate I (10g) was dissolved in THF (50mL), - Add dropwise to the reaction system at 5°C for 60 minutes. After the dropwise addition, keep warm for 2 hours and control the liquid phase. The remaining raw materials are less than 1%. Add methanol (10mL) to quench, concentrate the solve...
Embodiment 2
[0037]Preparation of Intermediate I: Add 10g of α-bromoacetophenone, 8.3g of 4-nitrophenethylamine, 41g of potassium carbonate, and 75mL of 1,4-dioxane into a 250mL three-neck flask, react at 90°C for 4h, and cool After reaching room temperature, filter, concentrate the solvent in vacuo to obtain an oil, add 25mL of petroleum ether to crystallize for 12h, filter, wash with 10mL of petroleum ether, and dry to obtain 9.6g of white solid, Intermediate I, with a yield of 68% and a purity of 99.40%.
[0038] Preparation of intermediate II hydrochloride: nitrogen protection, -5 ° C, BH 3 -DMS (1M, 70mL) was added dropwise to CBS (1M, 3.6mL,) for 30 minutes, and stirred for 60 minutes after the addition, Intermediate I (10g) was dissolved in THF (50mL), 0 ℃ was added dropwise to the reaction system, the dropping time was 60 minutes, the dropwise addition was completed and the reaction was incubated for 2 hours, the liquid phase was controlled in the middle, and the remaining raw mate...
Embodiment 3
[0040] Preparation of Intermediate I: Add 20g of α-bromoacetophenone, 18g of 4-nitrophenylethylamine, 41g of potassium carbonate, and 150mL of DMF into a 250mL three-neck flask, react at 60°C for 6h, cool to room temperature, filter, and concentrate the solvent in vacuo The oil was obtained, crystallized by adding 50mL of petroleum ether for 12h, filtered, washed with 20mL of petroleum ether, and dried to obtain 20g of white solid, Intermediate I, with a yield of 70.4% and a purity of 99.40%.
[0041] Preparation of intermediate II hydrochloride: nitrogen protection, -10°C, BH 3 -THF (1M, 70mL) was added dropwise to CBS (1M, 3.6mL,) for 30 minutes, and stirred for 60 minutes after the addition, and intermediate I (10g) was dissolved in THF (50mL), - Add it dropwise to the reaction system at 10°C for 60 minutes. After the dropwise addition, keep it warm for 2 hours and control the liquid phase. The remaining raw materials are less than 1%. Add methanol (10mL) to quench, concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com