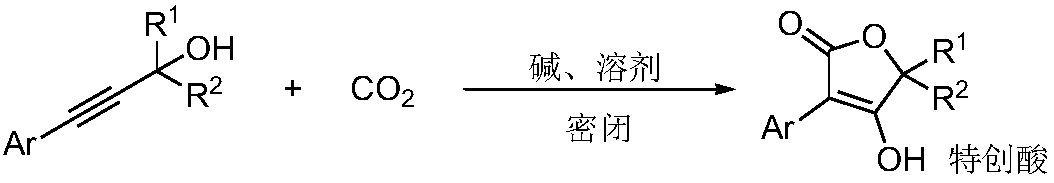
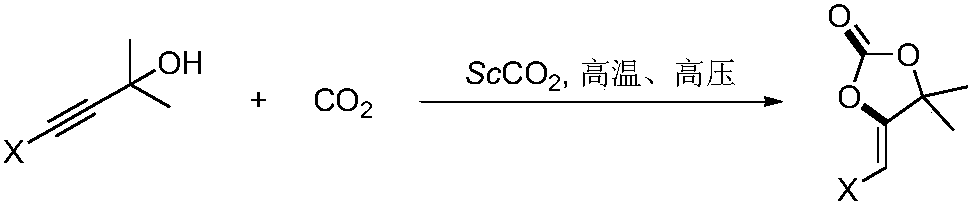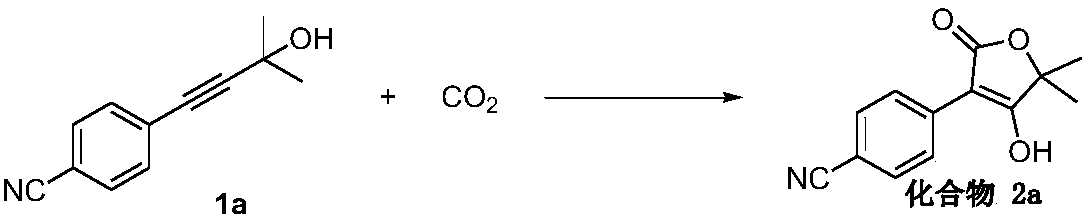Method for preparing tetronic acid by cyclisation and enolization of propargyl alcohol and carbon dioxide
A carbon dioxide, propargyl alcohol technology, applied in the chemical field, can solve the problems of harsh reaction conditions, unfavorable ionic liquid conditions for industrialized large-scale production, catalyst stability and reaction selectivity carbene instability, etc., and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of 5,5-dimethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2a)
[0039]
[0040] Accurately measure 4-(4-cyano-phenyl)-2-methyl-3-butyn-2-ol 1a (74.1 mg, 0.4 mmol) into the sealed tube, and weigh K under nitrogen 2 CO 3 (442.3 mg, 3.2 mmol), 2 mL of acetonitrile was added, and carbon dioxide was introduced. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 84.4 mg (0.368 mmol) of a white solid product with a yield of 92%. .
[0041] 1 H NMR (400MHz, DMSO-d 6 ) δ8.39~8.33 (m, 2H), 7.68~7.56 (m, 2H), 1.35 (s, 6H).
Embodiment 2
[0042] Example 2 Synthesis of 5-methyl-5-ethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2b)
[0043]
[0044] Accurately measure 4-(4-cyano-phenyl)-3-methyl-4-pentyn-3-ol 1b (79.7mg, 0.4mmol) into the sealed tube, weigh DBU (487.2mg, 3.2mmol), add 3mL DMF (N,N-dimethylformamide), and pass through carbon dioxide. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 82.7mg (0.340mmol) of a white solid product with a yield of 85% .
[0045] 1 H NMR (400MHz, DMSO-d 6 )δ8.47(d, J=8.4Hz, 2H), 7.49~7.47(m, 2H), 1.56(ddq, J=28.4, 14.3, 7.2Hz, 2H), 1.19(s, 3H), 0.71(t , J=7.4Hz, 3H).
Embodiment 3
[0046] Example 3 Synthesis of 5,5-diethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2c)
[0047]
[0048] Accurately measure 4-(4-cyano-phenyl)-3-methyl-4-pentyn-3-ol 1c (85.3 mg, 0.4 mmol) into the sealed tube, weigh CsF (486.1 mg, 3.2 mmol), 5 mL of DMI (1,3-dimethylimidazolidinone) was added, and carbon dioxide was introduced. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 77.2 mg (0.300 mmol) of a white solid product with a yield of 75%. .
[0049] 1 H NMR (400MHz, DMSO-d6) δ8.18 (d, J = 8.2Hz, 2H), 7.79 (d, J = 8.2Hz, 2H), 1.86 (dq, J = 14.9, 7.2Hz, 4H), 0.73 (t, J=7.3Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com