Preparation of 9,9-bis(6-hydroxynaphthalene-2-yl) fluorene crystal without complex residual solvent
A technology for residual solvents and hydroxynaphthalene, which is applied in the field of preparation of 9,9-bis(6-hydroxynaphthalene-2-yl)fluorene crystals without complexing residual solvents, and can solve difficult to implement, high melting point, high temperature melting products Problems such as easy discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
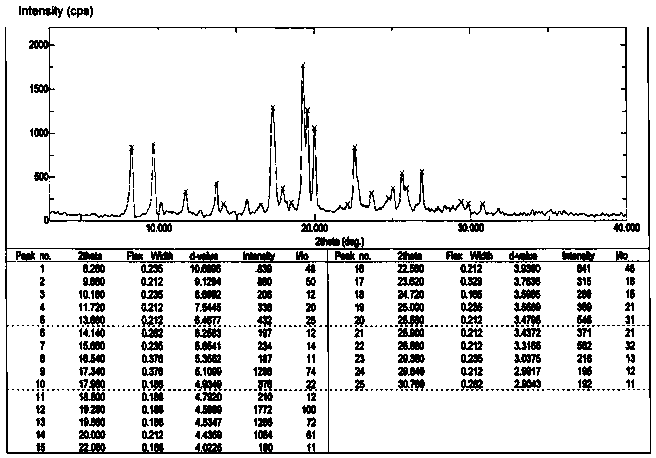
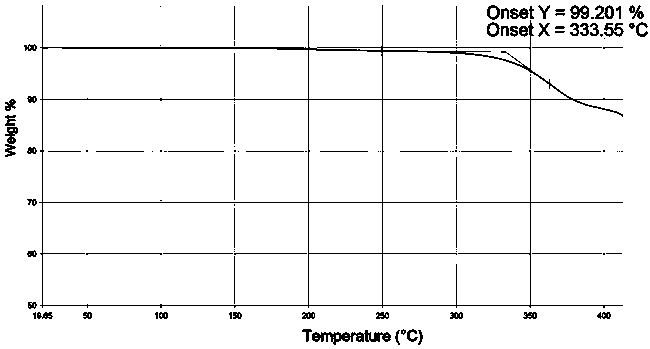
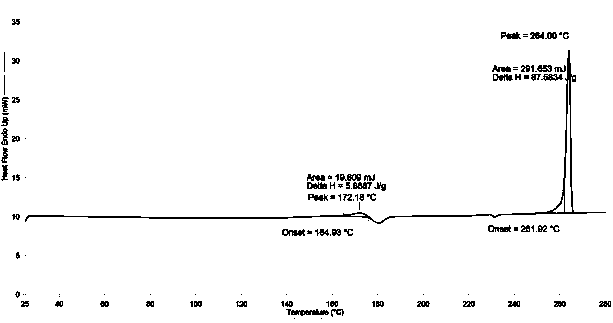
[0027] Add 9-fluorenone (18g, 0.1mol), 2-naphthol (33.1g, 0.23mol), 3-mercaptopropionic acid (1.0g), toluene 200 mL into the reaction flask, stir to dissolve and slowly add 98 % sulfuric acid 20.2g (0.2 mol). The reaction was stirred at 50-60°C for 4-8 hours. HPLC analysis confirmed that the 9-fluorenone content was below 0.1% and the reaction was stopped. Add 10% sodium hydroxide to neutralize, wash with 3*100mL water at 80°C. Separate the organic layer, evaporate the solvent under reduced pressure, add 100 mL of methanol, and stir to precipitate crystals. Use 1-chloropropane to recrystallize, and lower the temperature to 0°C under stirring at a speed of 0.3-1.0°C / min to crystallize. The crystals were filtered out, and vacuum-dried at 100°C / 1.33kPa for 8 hours to obtain 33.8 g of crystals, with a yield of 75.0% and a purity of 99.3% by HPLC analysis. 1 H NMR (500MHz, CDCl 3 )δ: 7.00-7.12(m, 4H), 7.37-7.41(m, 2H),7.51-7.57(m, 14H), 7.82-7.97(d, 2H). TGA spectrum (see fig...
example 2
[0029] The crude product was synthesized by the same steps, and then recrystallized with 1,2-dichloroethane, and crystallized at a rate of 0.3-1.0°C / min to 0°C while stirring. The crystals were filtered out and vacuum-dried at 100°C / 1.33 kPa for 8 hours to obtain 36.5 g of crystals with a yield of 81.0% and a purity of 99.5% by HPLC analysis. The X-powder diffraction spectrum shows that it has the same crystal form as Example 1.
example 3
[0031] The crude product was synthesized by the same steps, and then recrystallized with chloroform, and the temperature was lowered to 0 ℃ under stirring at a rate of 0.3-1.0 ℃ / min for crystallization. The crystals were filtered out and vacuum-dried at 100° C. / 1.33 kPa for 8 hours to obtain 33.5 g of crystals, with a yield of 74.5 % and a purity of 99.2 % by HPLC analysis. The X-powder diffraction spectrum shows that it has the same crystal form as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com