A kind of developable alginic acid-based biomaterial and its preparation method
A technology of biomaterials and alginic acid, which can be used in the preparation of X-ray contrast agents, drug delivery, and pharmaceutical formulations, and can solve problems such as limited indications, poor biological safety, and difficult surgical operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Put the developable alginic acid-based biomaterial that has been fully mixed according to the preparation method provided by the present invention in a cylindrical cavity mold, and after the developable alginic acid-based biomaterial sample is fully gelled, it is made into a diameter of 20 mm and a height of 20 mm. For a 20mm cylindrical sample, use a universal testing machine to test the support strength of the sample, set the pressurization speed to 2mm / min, and the clamping distance to 20mm, and obtain the support strength of the sample directly from the data output by the universal testing machine.
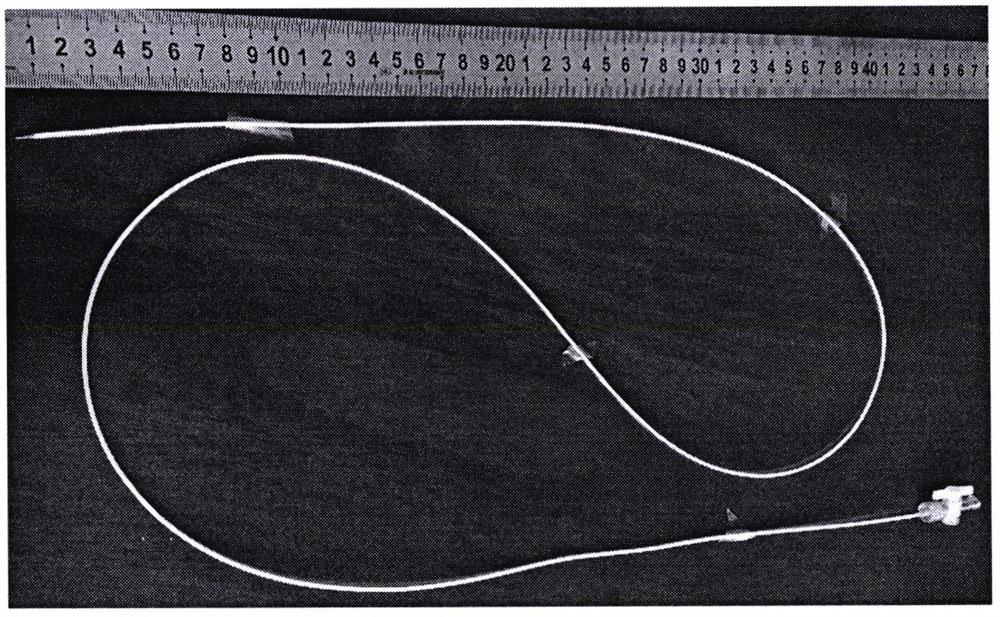
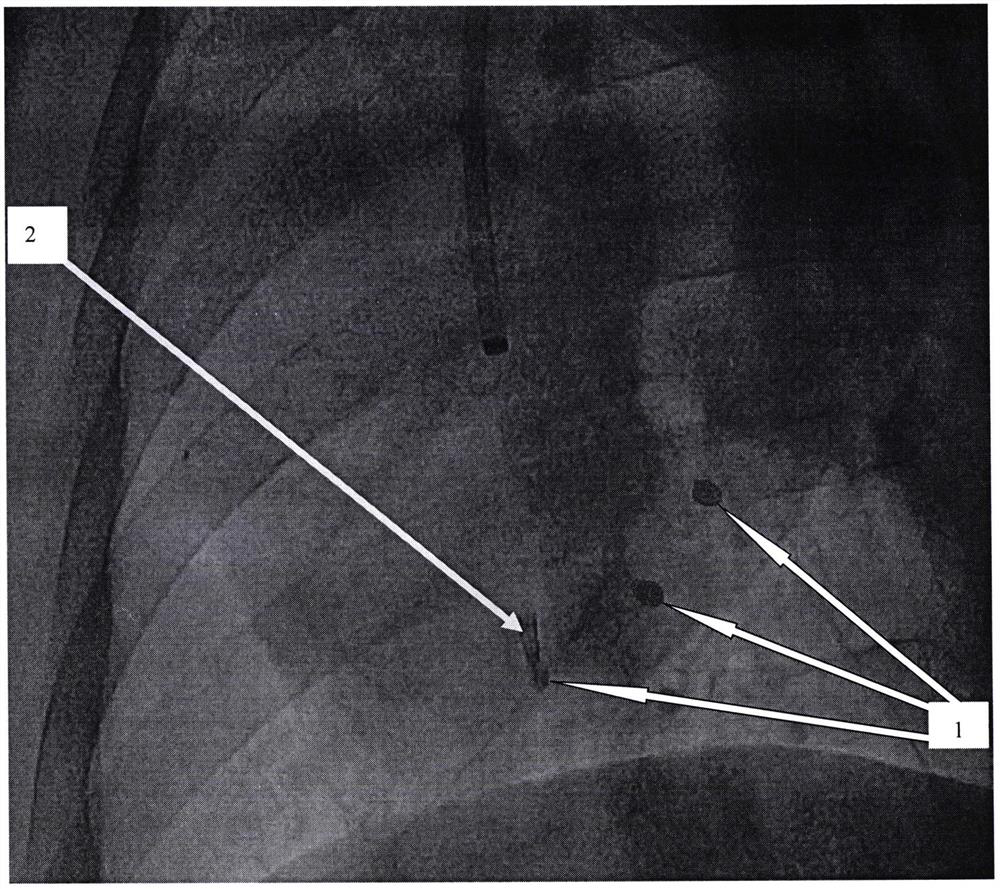
[0054] Multiple target injection experiment of minimally invasive vascular intervention surgery injection implantation:
[0055] The developable alginic acid-based biological material that has been fully mixed according to the preparation method provided by the present invention is subjected to animal experiments. The animal experiment object is a pig, and the femoral ...
Embodiment 1
[0070] (1) Premixing stage
[0071] (1) Preparation of the premix containing component B, component C and component D (ie: crosslinking agent + developer system): weigh the calculated amount of component B (calcium alginate, solid particle size is 75μm), component C (iohexol) and component D (mannitol) these three powders are dissolved together in water for injection, fully dissolved so that component B, component C and component D are in this premix The contents are 1.5% (g / ml), 75.5% (g / ml) and 302mmol / L respectively, and the pre-mixture is formed into a water-soluble solution with a pH value of 3.9.
[0072] (2) Preparation of the premix containing component A and component D (ie: sodium alginate system): weigh the calculated amount of component A (sodium alginate, molecular weight 180kDa) and component D (mannitol) These two powders are dissolved together in water for injection, fully dissolved, so that the contents of component A and component D in this premix are respec...
Embodiment 2
[0077] (1) Premixing stage
[0078] (1) Preparation of the premix containing component B, component C, component D and component E (ie: crosslinking agent + developer system): weigh the calculated amount of component B (calcium alginate, solid The particle size is 75 μm), the three powders of component C (iohexol) and component D (mannitol) are dissolved together in water for injection, fully dissolved, so that component B, component C and component D are in The contents of this premix are 1.5% (g / ml), 75.5% (g / ml) and 302 mmol / L respectively, and finally sodium hydroxide is used as component E regulator to increase the pH value of the premix from 3.9 To 7.0, the final premix is a water-miscible solution.
[0079] (2) Same as (2) described in Example 1.
[0080] (2) Reaction stage
[0081] (3) Same as (3) described in Example 1.
[0082] (4) Stable biomaterials can be formed after equilibrating for 6.17 minutes. The contents of component A, component B, component C and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com