Fluconazole tablets and preparation method thereof
A fluconazole tablet and the fluconazole tablet technology are applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problem of poor compressibility and friability of dispersible tablets. , the dissolution rate and bioavailability are not high, the tablet is easily broken, etc., to achieve the effect of stable appearance, high bioavailability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
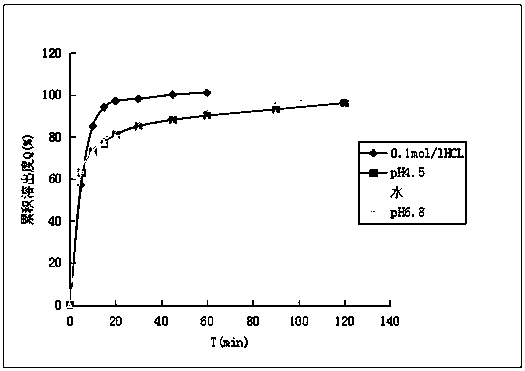
Embodiment 1
[0029] The oval fluconazole tablet of the present invention is composed of the following raw materials in parts by weight: 40 parts of fluconazole, 0.1 part of sodium alginate, 5 parts of anhydrous calcium hydrogen phosphate, 10 parts of microcrystalline cellulose, croscarmellose 2 parts of sodium cellulose, 5 parts of povidone K30, and 0.2 parts of magnesium stearate.
[0030] The preparation method of above-mentioned fluconazole tablet comprises the steps:
[0031] 1) Grind the raw and auxiliary materials in the prescription separately and pass through a 50-mesh sieve for later use;
[0032] 2) Weigh the raw and auxiliary materials in step 1) according to the prescription amount, take 2.5 parts of croscarmellose sodium, and then stir evenly;
[0033] 3) Then use purified water (the amount is 20% of the total mass of the prescription) to mix with the raw materials in step 2) evenly, the mixing time is 5 minutes, carry out wet granulation, pass through a 24-mesh sieve, and th...
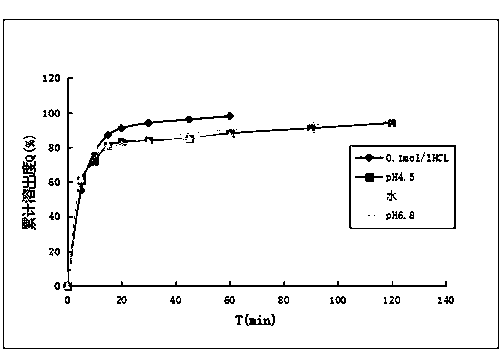
Embodiment 2
[0036] The oval fluconazole tablet of the present invention is composed of the following raw materials in parts by weight: 50 parts of fluconazole, 5 parts of sodium alginate, 15 parts of anhydrous calcium hydrogen phosphate, 20 parts of microcrystalline cellulose, croscarmellose 15 parts of cellulose sodium, 10 parts of povidone K30, and 2 parts of magnesium stearate.
[0037] The preparation method of above-mentioned fluconazole tablet comprises the steps:
[0038] 1) Grind the raw and auxiliary materials in the prescription separately and pass through a 50-mesh sieve for later use;
[0039] 2) Weigh the raw and auxiliary materials in step 1) according to the prescription amount, take 5 parts of croscarmellose sodium, and then stir evenly;
[0040] 3) Then use ethanol (the amount is 40% of the total mass of the prescription) to mix with the raw materials in step 2) evenly, and the mixing time is 15 minutes, carry out wet granulation, pass through a 24-mesh sieve, and then d...
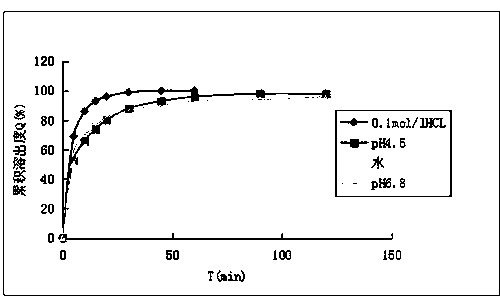
Embodiment 3
[0043] The oval fluconazole tablet of the present invention is composed of the following raw materials in parts by weight: 45 parts of fluconazole, 1.34 parts of sodium alginate, 10 parts of anhydrous calcium hydrogen phosphate, 15 parts of microcrystalline cellulose, croscarmellose 10 parts of sodium cellulose, 7 parts of povidone K30, and 1 part of magnesium stearate.
[0044] The preparation method of above-mentioned fluconazole tablet comprises the steps:
[0045] 1) Grind the raw and auxiliary materials in the prescription separately and pass through a 50-mesh sieve for later use;
[0046] 2) Weigh the raw and auxiliary materials in step 1) according to the prescription amount, take 5 parts of croscarmellose sodium, and then stir evenly;
[0047] 3) Then use ethanol (the amount is 20% of the total mass of the prescription) to mix with the raw materials in step 2) evenly, and the mixing time is 10 minutes, carry out wet granulation, pass through a 24 mesh sieve, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com