Cefovecin suspension injection and preparing method
A technology for suspension injection and cefotaxime, which is applied in liquid transportation, pharmaceutical formula, emulsion transportation, etc. It can solve the problems of unstable chemical properties, limited clinical application, insoluble in water, etc., and achieve easy preservation and dissolution Good effect and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of cefotaxime suspension injection
[0027] The present embodiment is a kind of cefotaxime suspension injection, and it carries out successively according to the following steps:
[0028] (11) Take 0.8 g of cefotaxime and pulverize it until 90% of the particles have a particle size of ≤20 μm, and then sterilize to obtain A1;
[0029] (12) Take 0.1g of antioxidant vitamin E and 1g of suspending agent glyceryl monostearate and sterilize to obtain B1;
[0030] (13) Add soybean oil, the solvent for injection, to 100 mL, heat to 65°C, keep warm for 20 minutes, sterilize at 120°C for 10 minutes, and then cool to room temperature to obtain C1;
[0031] (14) Add A1 and B1 to C1 in turn, stir at a speed of 120r / s and completely disperse into a suspension;
[0032] (15) The suspension in step (14) was homogenized at 6.5 MPa for 5 minutes to obtain a finished product.
[0033] The cefotaxime suspension injection prepared in this example has stable and unifor...
Embodiment 2-5
[0034] Example 2-5 Cefotaxime Suspension Injection
[0035] This example is respectively a kind of cefotaxime evening suspension injection, and the preparation method is similar to that of Example 1, the only difference is that the corresponding technical parameters in the preparation process are different, as shown in the following table. In the following examples, soybean oil, the solvent for injection, was added until the total solution volume reached 100 mL.
[0036]
[0037] The cephalosporin suspension injection prepared in Examples 2-5 has stable and uniform properties, is convenient to use, is easy to store, and has a long shelf life; the preparation method is simple, the process is easy to control, and the cefotaxime dissolving effect is good, which is suitable for industrial production. .
Embodiment 6
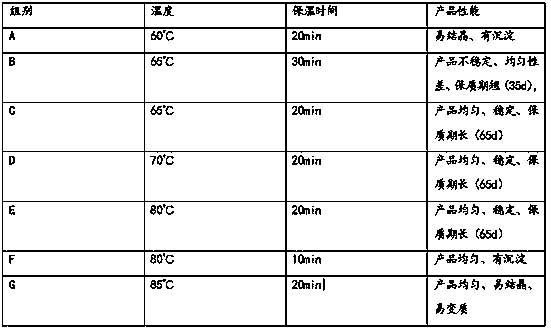
[0038] Example 6 Influence of the heating temperature and holding time of the solvent for injection during the preparation of cefotaxime suspension injection on product performance
[0039] In the preparation process of cefotaxime suspension injection, the heating temperature and holding time of the solvent for injection are very important. The heating temperature and holding time of soybean oil can ensure that the soybean oil will not be oxidized and deteriorated, and it can be used within a certain energy range. Inside, the particles are evenly dispersed in the soybean oil, and the particles are wrapped so that the particles will not agglomerate or float. At this time, whether the soybean oil can wrap the particles and the thickness and diameter of the wrap are related to the suspension formed by the final dispersion. Stability and uniformity are closely related. In order to explore the influence of soybean oil heating temperature and holding time on the performance of the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com