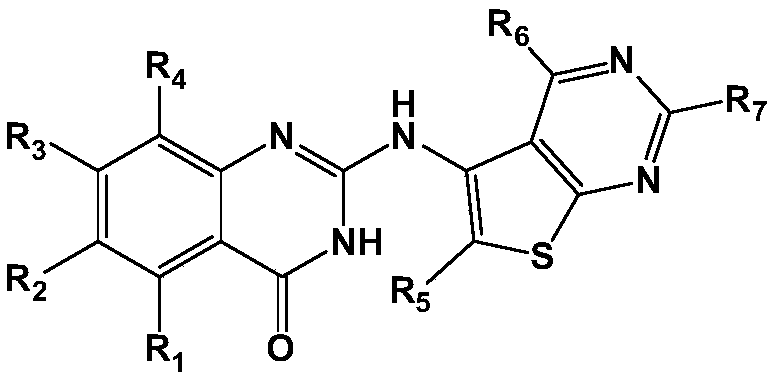
Compounds and uses thereof for treating atherosclerosis
A compound and composition technology, applied in the field of medicine, can solve the problems of niacin patients' compliance troubles, side effects, flushing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0059] Hereinafter, the present invention is described in more detail to facilitate understanding of the present invention.
[0060] Those skilled in the art will recognize that the chemical reactions described herein can be used to suitably prepare many other compounds of the invention and that other methods for preparing the compounds of the invention are considered to be within the scope of the invention Inside. For example, the synthesis of those non-exemplified compounds according to the present invention can be successfully accomplished by those skilled in the art through modification methods, such as appropriate protection of interfering groups, by using other known reagents in addition to those described in the present invention, or by incorporating Reaction conditions with some routine modifications. In addition, reactions disclosed herein or known reaction conditions are also recognized to be applicable to the preparation of other compounds of this invention.
Embodiment 1
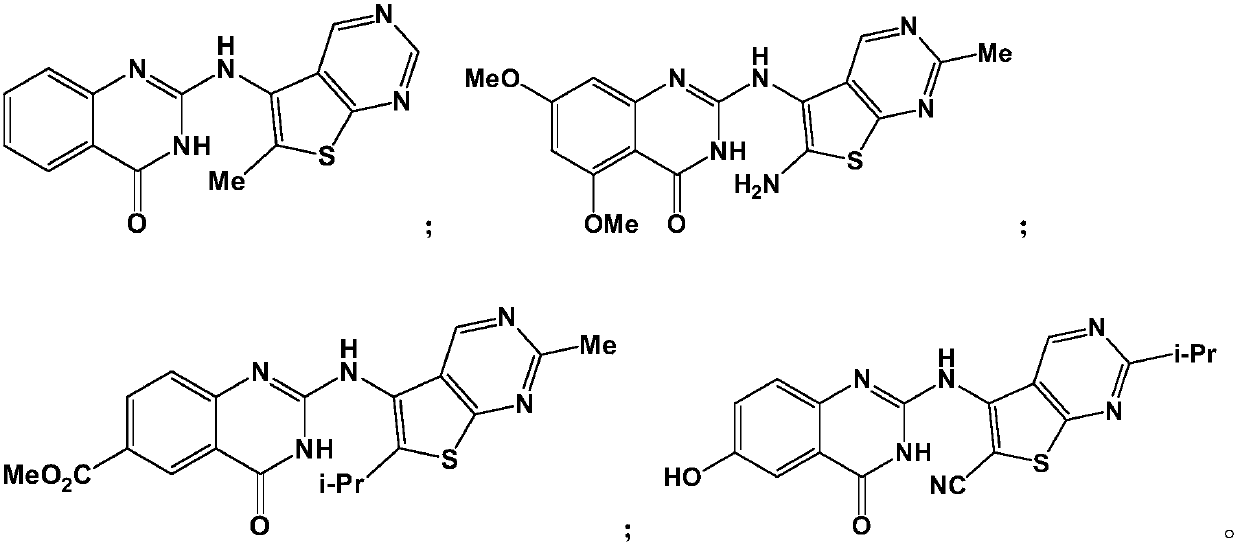
[0061] Example 1: 2-(6-methylthien[2,3-d]pyrimidin-5-ylamino)quinazolin-4(3H)-one (compound TPQZ-1)
[0062]
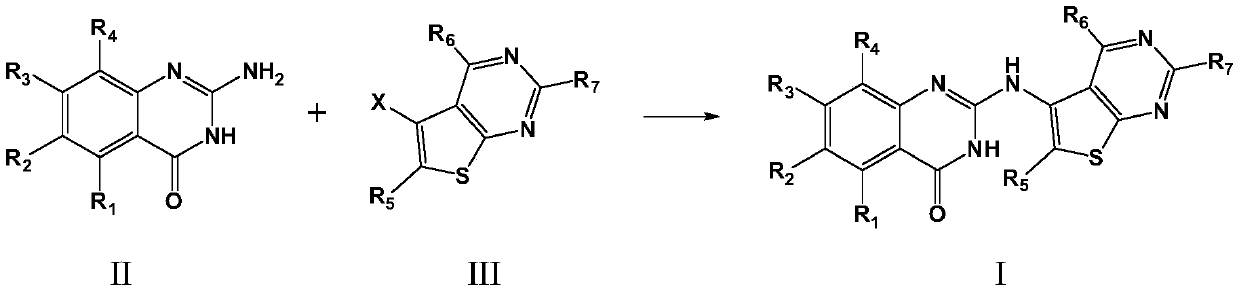
[0063] Add 2-aminoquinazolin-4(3H)-one (5.0mmol), 5-bromo-6-methylthien[2,3-d]pyrimidine (5.0mmol) to 50mL N,N-dimethyl Add 60% NaH (10.0mmol) to the above mixture in batches under stirring in formamide, and add 60% NaH (10.0mmol) in batches to room temperature, then continue the reaction for 5 hours. After the reaction is complete, add 2 mL of glacial acetic acid to terminate the reaction . The reaction solution was poured into 100 mL of saturated sodium bicarbonate solution, the precipitated solid was filtered, washed with water, and the filter cake was dried and recrystallized with ethyl acetate to obtain 1.22 g of the target product with a yield of 79.5%.
[0064] Mass Spectrum (ESI): 310.35[M+H] +
[0065] Elemental analysis: theoretical value C, 58.24; H, 3.58; N, 22.64; O, 5.17; S, 10.37
[0066] Found value C, 58.15; H, 3.78; N, 22.48; O, 5.06; S, 10.53...
Embodiment 2
[0068] Example 2: 2-(6-amino-2-methylthiophene [2,3-d]pyrimidin-5-ylamino)-5,7-dimethoxyquinazolin-4(3H)-one ( Compound TPQZ-2)
[0069]
[0070] 2-amino-5,7-dimethoxyquinazolin-4(3H)-one (5.0mmol), 5-bromo-2-methylthien[2,3-d]pyrimidin-6-amine ( 5.0mmol) was added to 50mL N,N-dimethylformamide, cooled to 0°C in an ice bath, and 60% NaH (10.0mmol) was added to the above mixture in batches under stirring, and the temperature was naturally raised to room temperature, and then continued The reaction was carried out for 7 hours. After the reaction was complete, 2 mL of glacial acetic acid was added to terminate the reaction. The reaction solution was poured into 100 mL of saturated sodium bicarbonate solution, the precipitated solid was filtered, washed with water, and the filter cake was dried and then recrystallized with toluene to obtain 1.43 g of the target product with a yield of 74.6%.
[0071] Mass Spectrum (ESI): 385.41[M+H] +
[0072] Elemental analysis: theoretica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com