Preparation method of polynorbornene skeleton sugar polymer and its application in the synthesis of fucoidan sulfate mimics
A technology of fucoidan sulfate and polynorbornene, which is applied in the field of polymer material synthesis and preparation, and achieves the effects of large molecular weight, simple preparation method, and narrow molecular weight distribution range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
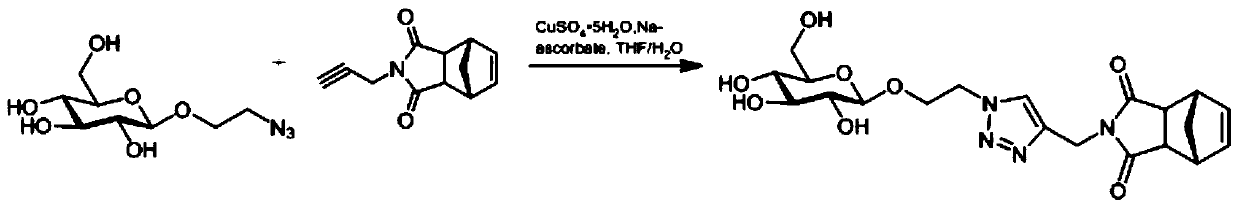
[0036] Embodiment 1: the preparation of glucose monomer
[0037] Such as figure 1 As indicated, weigh 110.0 mg (0.44 mmol) of azide ethanolated glucose in a round bottom flask, add 4 ml THF / H 2 Dissolved in O (1:1), add 138.8mg (0.66mmol) of propargyl aminated exo-norbornene anhydride, add 868.3μl (0.088mmol, 25mg / ml) of copper sulfate pentahydrate, and 929.9μl of sodium ascorbate ( 0.528mmol, 100mg / ml), react at room temperature for 12h, evaporate the reaction solution to dryness, and obtain pure glucose monomer by column chromatography.
Embodiment 2
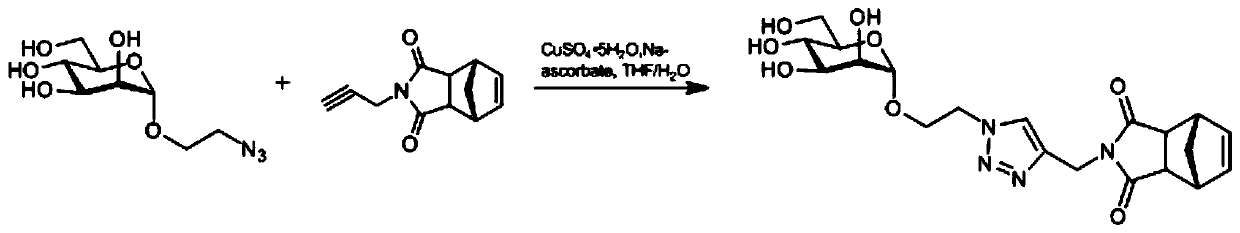
[0038] Embodiment 2: the preparation of mannose monomer
[0039] Such as figure 2 As indicated, weigh 110.0 mg (0.44 mmol) of azide ethanolated mannose in a round bottom flask, add 4 ml THF / H 2 Dissolved in O (1:1), add 38.8mg (0.66mmol) of propargyl-aminated exo-norbornene anhydride, add 868.3μl (0.088mmol, 25mg / ml) of copper sulfate pentahydrate, and 929.9μl of sodium ascorbate ( 0.528mmol, 100mg / ml), react at room temperature for 12h, evaporate the reaction solution to dryness, and obtain pure mannose monomer by column chromatography.
Embodiment 3
[0040] Embodiment 3: Preparation of polynorbornene-based glucose homopolymer (p-Glu)
[0041] Take 10 mg of pure glucose monomer in Example 1 in a microwave reaction tube, add 11 mg of phase transfer catalyst DTAB, add 600 μl of bis-Tris buffer and dichloroethane, add 174 μl of catalyst Hoveyda-Grubbs 2 nd , stirred at room temperature to form a stable emulsion state, placed in a microwave reactor for 5 minutes, added excess vinyl ether to terminate the reaction, and the reaction solution was directly separated and purified with LH-20, the eluent used was double distilled water, collected samples, double Distilled water dialyzed for 3 days, freeze-dried to obtain polynorbornene-based glucose homopolymer p-Glu, its H NMR spectrum is as follows: image 3 As shown, its molecular weight analysis spectrum is as Figure 4 shown. Depend on image 3 It can be seen that the chemical shift of the glucose anomeric hydrogen occurs around 4.25 ppm, proving the presence of glucose in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com