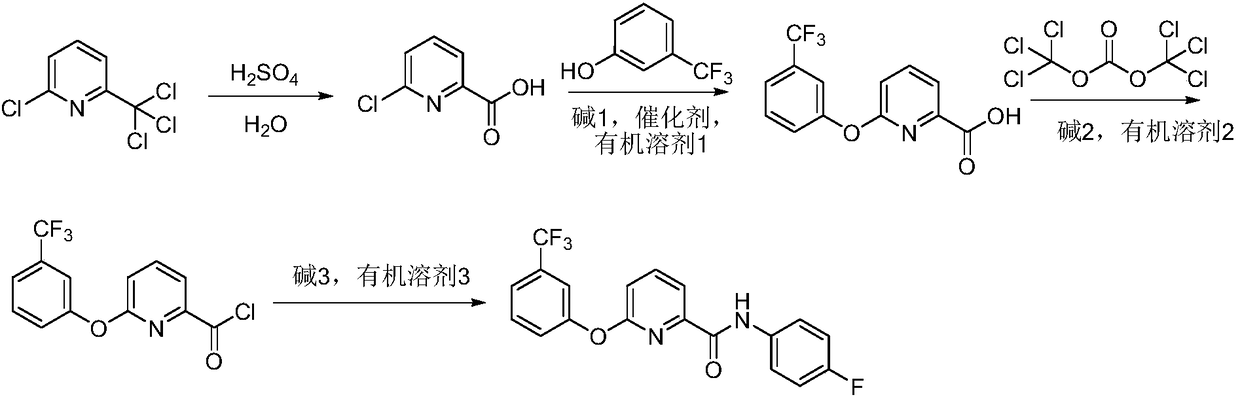
Method for preparing picolinafen
A technology of flufenamid and flufenamide, which is applied in the field of flufenamid preparation, can solve the problems of no flufenamid synthesis process, difficult effective separation and recycling of tail gas, and high equipment requirements. Achieve the effects of reducing discharge and waste water discharge, low equipment and operation requirements, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 2-chloro-6-trichloromethylpyridine (115.5g, 0.5mol) and 98% concentrated sulfuric acid (60g, 0.6mol) successively into a 500mL three-necked flask equipped with a thermometer, heat to 100°C, and react for 8.0h . After the reaction, the temperature was lowered to 60° C., and 27% ammonia water was added dropwise to neutralize to pH=7. After cooling to room temperature and stirring, a solid precipitated out and was suction filtered to obtain a white solid with a yield of 90%, m.p.: 192-193°C.
[0028] Add DMF (200ml) in the 500mL three-necked flask equipped with a thermometer, add m-trifluoromethylphenol (0.26mol, 42.2g) successively, potassium carbonate (0.266mol, 37.2g), 2-chloro-6-carboxypyridine (0.2mol, 31.6g), ketone chloride (1.0g), heated to 140°C, reacted for 6.0h, cooled to room temperature, poured the reaction solution into 600ml of ice water, adjusted the pH to 3 with concentrated brine, precipitated solid, pumped Filter, wash with water, and dry to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com