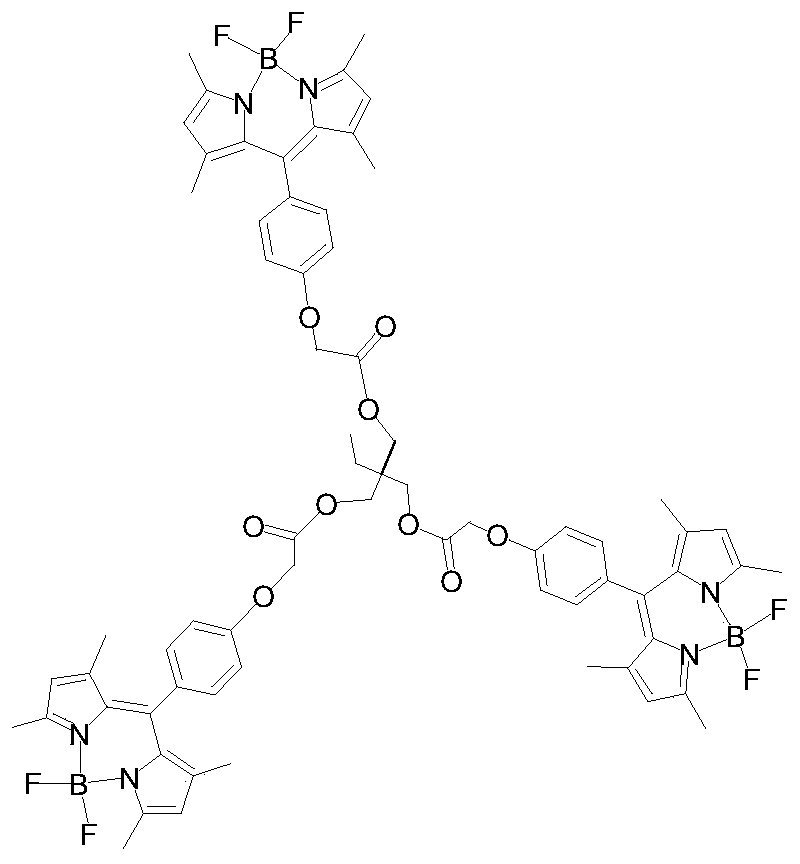
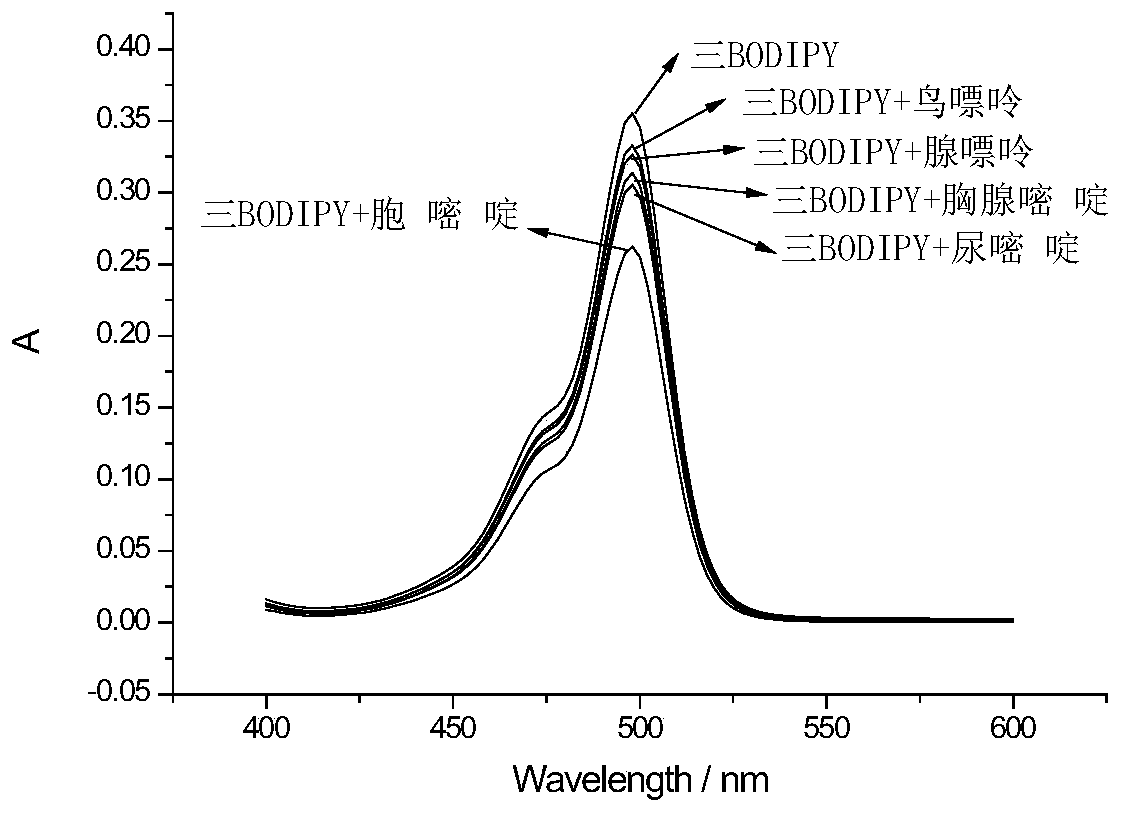
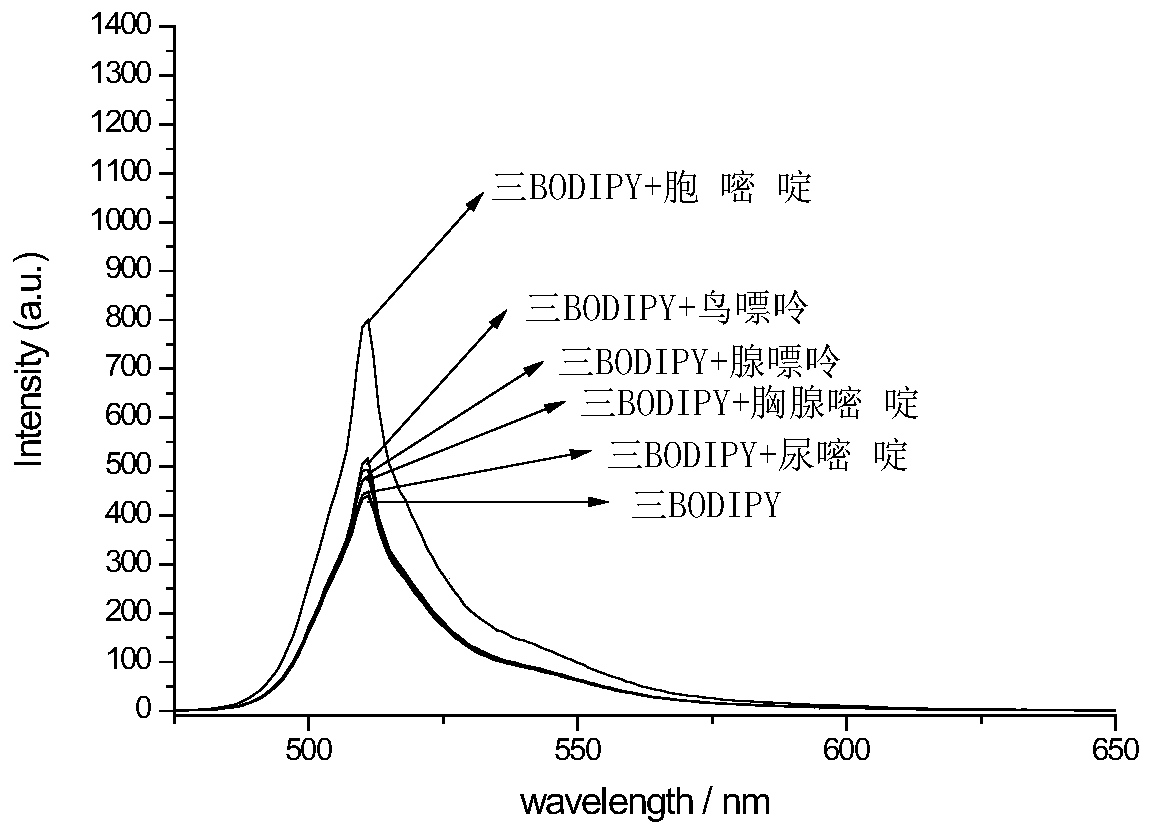
A fluorescent probe for detecting cytosine and its preparation method
A technology of fluorescent probe and cytosine, applied in the field of fluorescent probe for detection of cytosine and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. The synthetic steps of three (2-oxo-chloroethoxymethyl) propane:
[0031] Add 1.34g (0.01mol) of trihydroxypropane and 3.39g (0.03mmol) of chloroacetyl chloride into a 100mL three-neck flask filled with 25mL of dichloromethane, heat and stir under reflux for 4 hours to stop the reaction. Cool to room temperature, add 5% sodium hydroxide solution to fully wash the organic layer 3 times, separate the organic layer, dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain tris(2-oxo-chloroethoxymethyl) Propane solid, yield 85%.
[0032] 2. The synthesis steps of three BODIPY:
[0033] In a three-necked flask equipped with 50 mL of acetonitrile, add 0.363 g (0.001 mmol) of tris(2-oxo-chloroethoxymethyl) propane, 1.02 g of hydroxy BODIPY (0.003 mmol), and 1.0 g of potassium carbonate (0.007 mol) and potassium iodide 0.1g (0.0006mol), heated and stirred under reflux for 10 hours, and TLC detected that the raw material point disappear...
Embodiment 2
[0052] 1. The synthetic steps of three (2-oxo-chloroethoxymethyl) propane:
[0053] Add 1.34g (0.01mol) of trihydroxypropane and 5.65g (0.05mmol) of chloroacetyl chloride into a 100mL three-necked flask filled with 30mL of dichloromethane, heat and stir under reflux for 10 hours to stop the reaction. Cool to room temperature, add 5% sodium hydroxide solution to fully wash the organic layer 3 times, separate the organic layer, dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain tris(2-oxo-chloroethoxymethyl) Propane solid, yield 88%.
[0054] 2. The synthesis steps of three BODIPY:
[0055] In a three-necked flask equipped with 60mL of acetonitrile, add 0.363g (0.001mmol) tris(2-oxo-chloroethoxymethyl)propane, 1.36g (0.004mmol) of hydroxy BODIPY, 2.0g (0.014mol) of potassium carbonate and potassium iodide 0.2g (0.0012mol), heated and stirred under reflux for 12 hours, and TLC detected that the raw material point disappeared. Stop th...
Embodiment 3
[0057] 1. The synthetic steps of three (2-oxo-chloroethoxymethyl) propane:
[0058] Add 1.34g (0.01mol) of trihydroxypropane and 6.78g (0.06mmol) of chloroacetyl chloride into a 100mL three-necked flask filled with 40mL of dichloromethane, heat and stir under reflux for 5 hours to stop the reaction. Cool to room temperature, add 5% sodium hydroxide solution to fully wash the organic layer 3 times, separate the organic layer, dry over magnesium sulfate, filter, and evaporate the solvent under reduced pressure to obtain tris(2-oxo-chloroethoxymethyl) Propane solid, 82% yield.
[0059] 2. The synthesis steps of three BODIPY:
[0060] In a three-necked flask equipped with 50mL of acetonitrile, add 0.363g (0.001mmol) tris(2-oxo-chloroethoxymethyl)propane, 2.04g (0.006mmol) of hydroxy BODIPY, 3.0g (0.021mol) of potassium carbonate and potassium iodide 0.2g (0.0012mol), heated and stirred under reflux for 20 hours, and TLC detected that the raw material point disappeared. Stop the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com